ABSTRACT
Scinax belloni is a small bromeligenous treefrog that lives in the mountainous areas of southeastern Brazil. The call of the species has been previously described, but recently gathered data revealed an unreported acoustic unit that we describe herein. Intraspecific variation within the species call was also updated based on an increased sample size. The call is composed of two types of multipulsed notes: a short note, which is in agreement with the previous description, and a trill note that is emitted in association with the short note. The trill note was likely not reported in the previous description because of the small sample size. Further studies should elucidate the social function of the trill note. Acoustic units somewhat resembling the trill note are shared by a few other Scinax species, but the nomenclatural instability of these calls hamper detailed comparisons. This highlights the need for a standardized and comparative acoustic analysis to elucidate homologous and comparable acoustic units among the bromeligenous Scinax species.
Scinax Wagler, 1830 comprises more than 120 species that are distributed across the Neotropics from southern Mexico to Argentina and Uruguay [Citation1,Citation2]. Segalla et al. [Citation3], Colaço and Silva [Citation4], and Lourenço et al. [Citation5] considered the genus Ololygon Fitzinger, 1843 that was recently resurrected by Duellman et al. [Citation2] for the S. catharinae clade of Faivovich et al. [Citation1], as a junior synonym of Scinax. The S. catharinae clade harbors the S. perpusillus group, which is composed of species that use bromeliads for reproduction. These species are distributed throughout the coastal and mountainous areas of southeastern to southern Brazil, from the state of Espírito Santo (ES) to the state of Santa Catarina [Citation6].
Scinax belloni is a small (snout-to-vent length (SVL) of 19.8–23.0 mm [Citation6,Citation7]) bromeligenous treefrog only known from its type locality in the surroundings of the Parque Estadual (PE) do Forno Grande, municipality of Castelo, ES, southeastern Brazil. The species is characterized by a dorsum densely covered with granules and the lack of any coloration, mark, or blotch on the dorsum, inguinal region, or hidden surfaces of hind limbs. The call of the species was described previously [Citation7] from its type locality, but recently gathered data revealed an undescribed acoustic unit. Therefore, we reassessed the vocal repertoire of S. belloni with an increased male sample size, and described the additional acoustic unit.
Fieldwork was conducted between November 26 and 29, 2019, in the type locality of S. belloni, in the surroundings of the PE do Forno Grande (20°31ʹ0.16″ S, 41°5ʹ2.60″ W; 1120 m a. s. l.; WGS84 datum) within the municipality of Castelo (ES). Recordings were obtained with a digital recorder (Marantz PMD 671; 44.1-kHz sampling rate and 16-bit resolution) and Sennheiser ME66/K6 directional microphone. Voucher specimens and recordings were deposited in the Coleção de Anuros da Universidade Federal de Uberlândia (AAG-UFU) in Uberlândia, Minas Gerais, Brazil, under the following accession numbers: AAG-UFU 6770–71. Details of the sound files analyzed in Appendix 1. Collected specimens were identified as S. belloni based on the morphological traits cited above and the original description [Citation6]. The SVL of the specimens was measured with a digital Mitutoyo caliper to the nearest 0.1 mm as the distance line from the tip of the snout to the posterior margin of the vent.
Calls were analyzed using Raven Pro 1.5 [Citation8], with the following settings for spectrogram: window type = Hann, window size (FFT) = 512 samples, 3 dB filter bandwidth = 124 Hz, window overlap (locked) = 90%, hop size = 1.16 ms, discrete Fourier transform (DFT) size = 1024 samples, and grid spacing = 43.1 Hz. High-pass filters were applied below 200 Hz to reduce background noise caused by wind. Following the note-centered approach of Köhler et al. [Citation9], we measured the following acoustic traits: call duration, call rate, note number, note duration, pulse number per note, pulse rate (the ratio between the number of pulses and note duration), and note dominant frequency. Temporal traits were measured manually from oscillograms, while the spectral trait of dominant frequency was obtained using “Peak Frequency” measurement function of Raven. Sound figures were produced using the seewave v.1.7.6 [Citation10] and tuneR v.1.3.2 [Citation11] packages of R v.3.6.1 [Citation12], with window type = Hann, FFT overlap = 90%, and FFT size = 512 samples. Spectrogram figures were produced using a color scale, in which red represents the maximum amplitude (0 dB).
Individuals were found to vocalize inside leaf-tanks of terrestrial and rupicolous bromeliads. The SVL ranged from 19.4 to 24.2 ms (21.8 ± 3.4, n = 2 males; ). The call of S. belloni (n = 7 males; was composed of two distinct types of notes, both with multipulsed structures: a short note with pulses partially fused and a trill note that was often longer and represented by a train of pulses. Calls could be composed of only short notes without the trill, emitting 1–3 short notes (1.8 ± 0.4, n = 36); in this case, call duration ranged from 11 to 380 ms (67 ± 26, n = 36). Trill notes were always emitted just after 1–3 short notes (1.7 ± 0.4, n = 44); call duration of short notes + trills ranged from 431 to 1576 ms (930 ± 159, n = 44). Although calls composed of short notes + trills were generally more common (six out of seven males emitted trill notes) than calls with only short notes, one of the males emitted calls with only short notes for more than one minute. A call of an excited male was commonly composed of sequences of consecutive short and trill notes (). Each short note had a duration of 6–134 ms (33 ± 20, n = 138), and 1–40 pulses (9 ± 2.4, n = 138), which were commonly emitted with no silent interval between each pulse, except for the first pulses that tended to be separated. Pulses were emitted at highly variable rates of 79–667 pulses/s (336 ± 395, n = 138). The dominant frequency of short notes ranged from 2756 to 4608 Hz (3928 ± 395, n = 138; ). Trill notes had durations of 116–1009 ms (550 ± 136, n = 45), and were composed of 3–69 pulses (24.4 ± 16.4, n = 45), with silent intervals between each pulse. Pulses were arranged in groups throughout the trill duration. The pulse rate of the trill notes was also variable, ranging from 12 to 104 pulses/s (44 ± 26.9, n = 45), and the dominant frequency of trill notes ranged from 2713 to 4479 Hz (4009 ± 350, n = 45; ). The call rate (considering both short and trill notes) ranged from 39 to 57 calls/min (47 ± 8, n = 7). The interval between short notes ranged from 14 to 285 ms (88 ± 83, n = 61), and between short notes and trills was 36 to 317 ms (222 ± 44, n = 34). Intervals between calls ranged from 226 to 2627 ms (733 ± 226, n = 61).
Figure 1. (A) Adult male of Scinax belloni (AAG-UFU 6771, SVL = 19.4 mm). (B–C) The vocal repertoire of S. belloni from its type locality. (B) An oscillogram showing a sequence of 22 calls (note that two calls are emitted with only short notes in the beginning of this cut). (C) Spectrogram and respective oscillogram detailing the call highlighted in (A). (D) Power spectra of both notes depicted in (C). Sound file = Scinax_belloniCasteloES6aPM_AAGm671 (metadata of recording in Appendix 1)
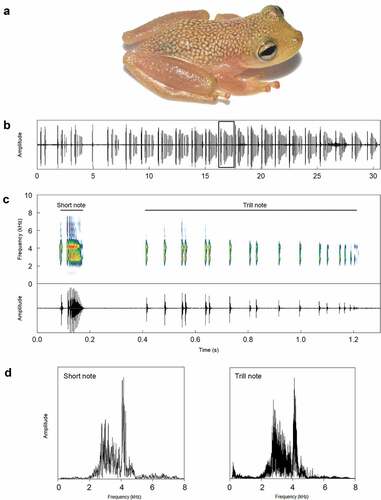
Although Peres and Simon [Citation7] did not report on the trill note, all of the acoustic traits reviewed for short notes fell within the range of variation provided by those authors, except for the dominant frequency, which tended to have higher mean values (i.e. 3928 Hz for short notes), even though the value (i.e. 3078 Hz) of Peres and Simon [Citation7] was within the range of this study. The trill note in the vocal repertoire of S. belloni is described herein for the first time. This note was not reported by Peres and Simon [Citation7], likely because of the small sample size (three males and 22 cals were analyzed). Other calling males in close bromeliads to the recorded individuals might have conditioned the motivational state of males to emit trill notes. Experimental and behavioral studies should elucidate the social function of short and trill notes in the repertoire of S. belloni [Citation13,Citation14], especially in terms of the trill note as a component of the advertisement call or in a courtship or aggressive/territorial role.
The calls of 8 of the 13 species within the Scinax perpusillus group species have been documented [Citation15], and an acoustic unit somewhat resembling the trill note of S. belloni appears to be shared with S. littoreus (i.e. “call B” [Citation16]) and S. v-signatus (i.e. “call II” [Citation15]). Although overlap exists between acoustic traits, the trill note of S. belloni (duration: 116–1009 ms; dominant frequency: 2713–4479) tended to have a longer duration and lower dominant frequency than “call B” of S. littoreus (220–349 ms; 5292–5439 Hz). “Call II” of S. v-signatus has a much longer duration of 47.8 s and tends to have a slighter higher dominant frequency (2812–4875 Hz). The presence of a trill note in the vocal repertoire of S. belloni should be useful in differentiating it from the other remaining bromeligenous species of the S. perpusillus group [Citation15–20]. Scinax arduous, S. cosenzai, S. perpusillus, S. peixotoi, and S. insperatus appear to only emit equivalents of the “short notes” of S. belloni, but additional sampling would likely reveal unreported acoustic units in their vocal repertoires as well.
There appears to be a nomenclatural instability of acoustic units among bromeligenous Scinax species (see discussion in Lacerda et al. [Citation20]); therefore, we did not compare acoustic traits other than call duration and dominant frequency between species. For example, Peixoto et al. [Citation15] and Pontes et al. [Citation16] considered aggressive calls of S. littoreus and S. v-signatus, respectively, as a series of multipulsed notes. Although pulse groups may be observed in the trill note of S. belloni, and one might assume that these groups should represent notes, we preferred to refer them as pulse groups as we observed isolated pulses being emitted within the span of the trill note. A standardized and comparative acoustic analysis should elucidate homologous and comparable acoustic units among the bromeligenous Scinax species.
Acknowledgments
Collection permits by ICMBio/SISBIO (#30059-12).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Faivovich J, Haddad CFB, and Garcia PCA, et al. Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bull Am Mus Nat Hist. 2005;294(1):1–240.
- Duellman WE, Marion AB, and Hedges B. Phylogenetics, classification, and biogeography of the treefrogs (Amphibia: Anura: Arboranae). Zootaxa. 2016;4104(1):1–109.
- Segalla MV, Berneck B, Canedo C, et al. List of brazilian amphibians. Herpetol Brasil. 2021;10(1):121–216.
- Colaço G, and Silva HR. On the type series of Scinax perpusillus (lutz & lutz, 1939) (Anura: Hylidae). Zootaxa. 2016;4154(2):193–196.
- Lourenço ACC, Zina J, and Catroli GF, et al. A new species of the Scinax catharinae group (Anura: Hylidae) from southeastern Brazil. Zootaxa. 2016;4154(4):415–435.
- Faivovich J, Gasparini JL, and Haddad CFB. A new species of the Scinax perpusillus group (Anura: Hylidae) from Espírito Santo, Brazil. Copeia. 2010;1(1):97–102.
- Peres J, and Simon JE. O canto de anúncio de Scinax belloni, Faivovich, Gasparini & Haddad, 2010 (Amphibia: Anura: Hylidae). Rev Cient Faesa. 2011;7(1):47–54.
- Center for Conservation Bioacoustics. Raven pro: interactive sound analysis software [software]. Version 1.6. Ithaca(NY): The Cornell Lab of Ornithology; 2014. Available from http://www.birds.cornell.edu/raven
- Köhler J, Jansen M, Rodriguez A, et al. The use of bioacoustics in anuran taxonomy: theory, terminology, methods and recommendations for best practice. Zootaxa. 2017;4251(1):1–124.
- Sueur J, Aubin T, Simonis C. Seewave, a free modular tool for sound analysis and synthesis. Version 1.7.6. Bioacoustics. 2008;18(2):213–226.
- Ligges U, Krey S, Mersmann O, et al. tuneR: analysis of music and speech. 2018. Available from: https://cran.r-project.org/package=tuneR.
- R Development Core Team. R A language and environment for statistical computing. Version 3. 6. 1. Vienna(AU): R Foundation for Statistical Computing; 2015. Available from http://www.R-project.org
- Narins PM, and Capranica RR. Communicative significance of the two-note call of the treefrog Eleutherodactylus coqui. J Comp Physiol. 1978;127(1):1–9.
- Bastos RP, Alcantara MB, and Morais AR, et al. Vocal behaviour and conspecific call response in Scinax centralis. Herpetol J. 2011;21:43–50.
- Peixoto MA, Guimarães CS, and Lacerda JVA, et al. Vocal repertoire of Scinax v-signatus (lutz, 1968) (Anura, Hylidae) and comments on bioacoustical synapomorphies for Scinax perpusillus species group. Acta Herpetol. 2016;11:53–57.
- Pontes R, Mattedi C, and Baêta D. Vocal repertory of Scinax littoreus (Anura: Hylidae) with comments on the advertisement call of the Scinax perpusillus species group. Zoologia. 2013;30(4):363–370.
- Pombal JP Jr., Bastos RP. Vocalizações de Scinax perpusillus (A. Lutz and B Lutz) e S. arduous Peixoto (Anura, Hylidae), com comentários taxonômicos. Rev Bras Zool. 2003;20(4):607–610.
- Silva HR, and Alves-Silva R. A new bromeligenous species of the Scinax perpusillus group from the hills of the State of Rio de Janeiro, Brazil (Anura, Hylidae). Zootaxa. 2011;3043(1):54–68.
- Brasileiro CA, Haddad CFB, and Sawaya RJ, et al. A new and threatened species of Scinax (anura: hylidae) from Queimada Grande Island, southeastern Brazil. Zootaxa. 2007;1391(1):47–55.
- Lacerda JVA, Peixoto OL, and Feio RN. A new species of the bromeligenous Scinax perpusillus group (Anura; Hylidae) from Serra do Brigadeiro, State of Minas Gerais, Southeastern Brazil. Zootaxa. 2012;3271:31–42.
Appendix
Appendix 1.
Analyzed sound files from the accessory bioacoustics repository of AAG-UFU with associated metadata (date, time at recording, air temperature, and voucher specimen).
Scinax_belloniCasteloES2aDLB_AAGm671 (28 November 2019; 17:42 h; 20.2 °C; AAG-UFU 6770));
Scinax_belloniCasteloES3aDLB_AAGm671 (28 November 2019; 18:10 h; 20.2 °C);
Scinax_belloniCasteloES4aDLB_AAGm671 (28 November 2019; 18:15 h; 20.2 °C);
Scinax_belloniCasteloES5aPM_AAGm671 (28 November 2019; 18:30 h; 20.2 °C);
Scinax_belloniCasteloES6aPM_AAGm671 (28 November 2019; 18:39 h; 20.2 °C);
Scinax_belloniCasteloES7aPM_AAGm671 (28 November 2019; 18:43 h; 20.2 °C; AAG-UFU 6771);
Scinax_belloniCasteloES8aIV_AAGm671 (28 November 2019; 18:15 h; 20 °C);
