ABSTRACT
We report on the discovery of a population related to the Blunt-headed Salamander (Ambystoma amblycephalum), a micro-endemic axolotl from Mexico scientifically confirmed only once since its original description in 1940, and now presumably extinct. In 2018, paedomorphic and metamorphosed adults, as well as clutches and larvae, were found in a cattle pond at Nahuatzen, Michoacán state, Mexico, ~60 km away from the type locality (Tacícuaro). Morphometric comparisons suggested high similarity with the type series of A. amblycephalum, while mitochondrial DNA barcoding (16S and control region) revealed close (but imperfect) matching to a reference sequence. We gathered data on life history and ecology of this population, which could be the only extant relic of A. amblycephalum. Its highly limited distribution and presumably low population density are hallmarks for a high risk of extinction, and alarms on the critical situation of many micro-endemic salamanders of Mexico, hence calling for immediate conservation actions.
Introduction
Mexico is the fifth most biologically diverse country worldwide, with exceptional amphibian diversity and endemism [Citation1]. Unfortunately, the Mexican herpetofauna is highly threatened by anthropogenic pressures such as massive losses of habitat [Citation2,Citation3]. This situation is characteristic of Urodela (Caudata), i.e. axolotls, newts and salamanders, and nearly 400 species are considered endangered [Citation1,Citation4–9]. Due to their secretive lifestyle however, field observations are scarce, and the conservation status of Mexican salamanders is thus difficult to assess. This issue is particularly true in the neotropical region, where the diversity of Urodela has been underestimated [Citation10]. One reason is that many species might be micro-endemics with highly specialized ecological niches [Citation9,Citation11]. Consequently, conservation programs are often lacking the most basic information about their natural history.
Tiger salamanders from the genus Ambystoma (Ambystomatidae) makes an interesting example of the above issues. This group was long considered as a single polytypic species, A. tigrinum, which featured the largest geographic distribution among North American urodeles. In the past two decades, molecular studies identified complex patterns of diversification, involving polyploidy and gynogenetic lineages [Citation12], and [Citation13] distinct species are now recognized, inhabiting a wide range of habitats [Citation14]. These salamanders are notorious for neoteny, i. e. the retainment of larval traits at the adult stage, engendering so-called “perrenibranchiate” forms (also known as “neotenic” of “paedomorphic”). Neoteny appears to have evolved multiple times [Citation15–17], and has been documented among several micro-endemic taxa distributed along the Trans-Mexican Volcanic Belt [Citation15,Citation16,Citation18,Citation19]. Although Ambystoma salamanders require aquatic environments for larval development (and neotenic adults), most species remain mostly terrestrial and have fossorial lifestyles. Primarily nocturnal, they are essentially observed during an explosive breeding season, when they emerge from underground burrows and migrate to fresh waterbodies [Citation9,Citation20]. Hence, many species remain poorly known to science, although they are facing high risks of extinction [Citation21].
Here we focus on one of these micro-endemics, Ambystoma amblycephalum Citation22, in northwest Michoacán, Mexico. This taxon has so far been reported only twice in the scientific literature: from its original description at Tacícuaro, the type locality [Citation22], and by a genetic sample taken a few kilometers west in Iratzio [Citation17]. It is presently classified as critically endangered (CR) by the IUCN Red List [Citation23], and receives a special protection status by the Mexican government [Citation24]. In fact, the species has not been observed for decades and is eventually considered extinct [Citation25], including by some IUCN officials. In this paper, we report on a search survey of A. amblycephalum in the Purépecha Plateau (Nahuatzen, Mexico) conducted in 2017–2018, with the purpose to extend knowledge on its local distribution, natural history, and conservation status. Specifically, we discovered and documented a population putatively related to A. amblycephalum, which might represent the last relic of this enigmatic species.
Materials & methods
Study area
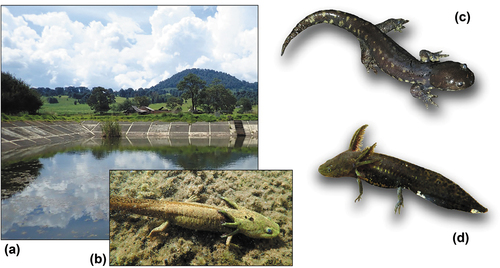
The surveyed area spanned 18 km2 comprising San Isidro up to Cheran townships, within the Purépecha Plateau, Nahuatzen Municipality in northwestern Michoacán state, Mexico (). It mainly encompasses a valley dominated by seasonal agriculture and livestock production. Land cover consists of shrubby vegetation, surrounded by hills of pine oak forests. Climate was described as “C(w2)” according to the Köppen Climate Classification subtype, i.e. temperate subhumid climate with annual average temperature between 12 and 18 ° C; winter rainfall representing 5–10% of the annual total. It is characterized by warm days and cool nights around the year, due to the high altitude of the plateau. In other words, the weather is wet/humid temperate, and most precipitation falls during the summer rainy season. Historical records and climatic data support this classification, e.g. minimum temperature (T° min) of 6.6 °C, average annual temperature (T° average/annual) of 14.8 °C, maximum temperature (T° max) of 25.2 °C, and total annual rainfall of 1070.15 mm [Citation26,Citation27]. To assess the local climatic conditions, monthly records from the weather station of Nahuatzen, and the regional Mexican offices of the SMN (National Weather Service), were extracted for the four-year period preceding our study (July 2014 to July 2018).
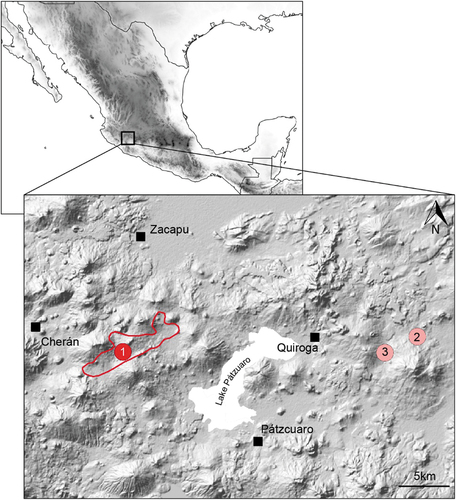
Figure 1. Area survey in this study (red), with approximate location of the discovered Nahuatzen population assigned to Ambystoma cf. amblycephalum in 2018 (marker 1), as well the two other scientifically documented records: the type locality of A. amblycephalum described by Citation22 (marker 2), and the origin of the genetic sample included in Citation17 (marker 3).
Field survey
We prospected for mole salamanders during several short field trips from November 2017 to August 2018, the longest one between 26 July 2017 and 9 August 2018. We applied the Visual Encounter Survey approach [VES, Citation28], a simple and time/cost effective method with little habitat disturbance, even for investigating large areas. Briefly, a target zone or habitat is searched systematically along delimited transect lines and time periods [Citation28]. The habitats surveyed included permanent and temporary streams, artificial reservoirs and irrigation canals, all searched by dip netting. Terrestrial surroundings were also investigated by removing stumps, stones and litter. Microhabitat characteristics were assessed, including land type, water and soil temperature, soil type, weather condition and vegetation structure. The vegetation was photographed with a Sony Nex-5 digital camera (Sony Ltd., Japan). Geographic data (coordinates, elevation) were collected in situ using a Global Positioning System (Garmin Montana 680; Garmin Ltd., Olahe, KS, USA). Water pH and temperatures were measured in situ using an Expresstech @ LCD PH Digital Meter (Expresstech; Kingpow Company Limited, Hong Kong, China).
Morphology
Adult Ambystoma specimens captured during the survey were measured at ten morphological parameters, as follows. SVL = snout-vent length (from the tip of the snout to the most posterior opening of the cloacal slit); TL = total length; TAL = tail length (from posterior edge of vent to tail tip); HL = maximum head length; HW = maximum head width; LAL = leg length; SL = snout length; ND = nostril diameter; IS = inter-nostril distance. All measurements were performed with a dial caliper (Louisware LSWCL1810, Louisware Ltd., USA; 0.1 mm precision). Individuals were subsequently released.
Our morphometric data was compared with the specimens documented in the original description of A. amblycephalum [Citation22], which listed three adult females: the holotype EHT-HMS N°16443 [now FMNH 100104 according to Citation29, see also Citation14] and the paratypes EHT-HMS N°16442 and EHT-HMS N°16444. All were collected 15 km west of Morelia, northern Michoacán state, Mexico on 10 September 1935 by E. H. Taylor. The general morphology of discovered clutches (egg and oocyte diameter) and captured larvae (SVL and TAL) were also assessed.
Molecular analysis
We performed genetic barcoding of one juvenile from the discovered population. The specimen was fixed in 96% ethanol and DNA was extracted using the Qiagen Blood & Tissue kit. Mitochondrial fragments from the 16S gene (~550bp) and the control region (CR, ~760) were amplified using the primers: 16SA (5’‐CGCCTGTTTATCAAAAACAT‐3’), 16SB (5’‐CCCGTCTGAACTCAGATCACG‐3’), Amby-CR-F1 (5’-CTTGTAAGTCGAAAACYGAAGAC-3’) and Amby-CR-R1 (5’-TATAATTWACTCATTCTATTTGG-3’). PCRs were carried out in 25 μl reactions containing 3 μl of template DNA, 12.5 μl of nanopure water, 7.5 μl of multiplex master mix (Qiagen, containing buffer, dNTPs and hot‐start polymerase) and 1 μl of each primer (10 μM), and were run as follows: 95°C for 15’, 35 PCR cycles of 94°C for 30’, 53°C for 45’ and 72°C for 1’; and 72°C for 5’. Amplicons were sanger-sequenced at GATC (Konstanz, Germany).
We first used BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to infer the identity of our Nahuatzen specimen based on the closest hits on GenBank sequences. Second, we harvested and concatenated 16S and CR sequences from published Ambystoma mitogenomes representing 12 different forms/taxa (AY659992–AY659995; AY728218, GU078469–GU078472; KP013120, KP289200). These were completed by a CR haplotype from our target species A. amblycephalum collected in Iratzio, which was independently obtained by two studies [U36403 from Citation17, DQ241132 from Citation30] – no equivalent data exist for 16S. Sequences were manually aligned and trimmed for analyses in Seaview [Citation31], and we obtained a concatenated alignment of 1,296 bp [16S: 541 bp, CR: 755 bp). Third, we aligned our Nahuatzen CR sequence with those from Citation32, which included 76 individuals of various Mexican Ambystoma.
Phylogenetic reconstructions of the two sequence aligments were performed in BEAST 2.6 (Citation30]. We applied strick clocks, a birth-death model for the tree prior, and GTR + G + I substitution models, which were those with the lowest AIC criteria for both genes [tested with jModelTest, Citation33]. The chains were run for 100 million iterations, sampling every 10,000 iterations. Stationarity and effective sample sizes of the parameters were visualized using Tracer [Citation34]. The first 20% of sampled trees were discarded as burnin, and we used DensiTree [Citation35] to visualize the retained trees, as well as the BEAST module TreeAnnotator to summarize them under the maximum-clade consensus method.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were strictly followed. All animal sample collection protocols complied with the current laws of Mexico. Specimen collection was conducted under the scientific permit SGPA/DGVS/05455/20 issued by the Secretaria de Medio Ambiente y Recursos Naturales (SEMARNAT) of Mexico.
Results
Discovered population
Seven adults (six paedormophic, one metamorphosed), three larvae and two clutches were discovered at Nahuatzen, Michoacán state, Mexico, all on 9 August 2018, around 2,500 m above the sea level (). The exact coordinates are not disclosed for conservation reasons. The site consists of cattle ponds located on a small plateau made up of basaltic lava in the Sierra de Nahuatzen ().
Morphometric assessments
Diagnosis of the Nahuatzen adult specimens
The captured adults feature light webbing and a slightly tuberculated skin, especially between the eyes and the snout. The head is longer than large. The limbs are long and robust. We counted 70 to 80 maxillary-premaxillary teeth (jaw teeth), and 11 costal grooves. Dorsal parts are blackish to olive-green, ventral parts (including the throat) are greyish and have variable sets of cream-colored spots. These cream-colored spots are less present in our adult females, as in the two paratypes of A. amblycephalum [Citation22]. The first half of the tail sides is lighter than the tail extremity, and displays small, irregularly arranged, dark brown spots. In paedomorphic specimens, we noted gills with reddish extremities (faded in older individuals). In fact, the overall body coloration was variable depending on the developmental stage (larvae, subadult, adult). The only metamorphosed specimen (WS2, an adult male) was found under a rotten pine tree and displayed a darker coloration, as also seen in the type series [Citation22]. Females were larger and more massive than males, growing up to 161.0 mm (TL) vs 128.8 mm in males. All morphometric measurements are available in .
Table 1. Measurements of adults related to Ambystoma amblycephalum (in mm). The first three compose the type series of the species [Citation22]; the following seven are the new specimens caught at Nahuatzen. F = female; M = male; L = larvae; SVL = snout-vent length; TL = total length; TAL = tail length; HW = head width; HL: head length; LAL: leg length; SL = snout length; ND = nostril diameter; IN = inter-nostril distance
Identification and comparative diagnosis
The adult individuals found in Nahuatzen were morphologically identified as A. amblycephalum (), according to the original description of Citation22, the most recent account by Citation9, and the comparative diagnosis below. The slightly tuberculated skin and coloration are typical, and A. amblycephalum further differs from related species by its body proportions, and in the greater number of jaw teeth.
Specifically, (i) A. bombypellum is smaller (in TL), differs in coloration, features twice less jaw teeth, and the vomero-palatine teeth are arranged differentially (separated by angular vomerine series); (ii) A. mexicanum differs in the general coloration, skin texture, and body proportions, notably the shape of the head, the general coloration and skin texture. (iii) A. flavipiperatum has more costal grooves (13), a smaller TAL/TL ratio, and in the color pattern (but both are similar in terms of vomerine teeth patterns); (iv) A. rosaceum is a larger species, has less vomerine teeth (~50), and a darker coloration; (v) A. lermaense has a different body shape (less compressed), lacks ventral cream-colored spots, and has less teeth; (vi) A. dumerilii has a different coloration (cream-colored spots on the dorsum) and 12 costal grooves; (vii) A. velasci shares many similarities, but is smaller and differs in coloration: it is darker (especially younglings) and the dorsal side lacks olive spots, but features presence cream-colored spots instead.
Larvae diagnosis
The examined larvae (n = 3) featured the following characteristics: head flat, labial fold slender, costal groove nearly indistinct. Long and blunt snout, large and blackish dorsal fin well present. General coloration dark blackish with irregular whitish to yellowish spots on the flanks. Gills are large and reddish, which is typical of pond-type larvae. Body and tail length are reported in .
Life history
Mating and egg deposition
Adults were all found in a single cattle pond (), hiding in the abundant algae cover (Spyrogyra sp.), but the courtship was not observed. Two large Ambystoma-like clutches were discovered in the same site and on the same day. Given that A. amblycephalum was the only Ambystoma identified in the area (see above), we can safely assume that these clutches belong to this taxon. Clutches comprised ~170 and ~220 eggs, deposited in single masses on the algae and the reed roots (Poaceae sp.). Most eggs featured a clear gelatinous layer, a few being slightly opaque. Egg diameter (including envelope) averaged 4.73 ± 0.55 mm (range 4.5–5.5), with a mean oocyte diameter of 1.98 ± 0.20 mm.
Habitat
All individuals were found in a cattle pond of approximately 30 × 30 m, filled with hard and stagnant water (pH = 9.43–9.45) of depths ranging from 32 to 59 cm. The pond’s bottom was covered with a thick layer of mud varying from 12 to 34 cm. Aquatic vegetation includes many Spirogyra spp. and some reeds that are used for egg deposition. Ponds were semitransparent with a high concentration of phytoplankton and zooplankton. Salamanders were observed together with aquatic insects and larvae such as Chironomus sp., Cyclops sp., Daphnia sp., Odonata and aquatic beetles, including metamorphosed and larval forms of Belostomatidae as well as Platambus mexicanus, which may be part of their diet.
The nearest terrestrial habitat is composed of loam and clay soils over lava flows, forming a pine oak forest land dominated by Muhlenbergia spp., Calamagrostis spp. Trisetum spp., Agrostis spp., Festuca spp. and other perennial grass. The area was surrounded by altered and scattered temperate subhumid mixed-pine and pine-oak forests, mainly composed of Pinus devoniana, P. pseudostrobus and P. leiophylla, as well as fir patches (Abies religiosa) at higher altitudes [>2600 m). According to Citation36, this complex composition of mixed forests is the result of human disturbance promoted by intensive logging and fire. Three specimens of Isthmura bellii were also observed under rocks in this area. According to the locals (the Purépecha indigenous group], the area has experienced extensive human modification. The pastoral industry (cow breeding) is the main activity, and the landscape is now dominated by agricultural fields and pastures. Indeed, the ponds of our field site were created to supply livestock with water.
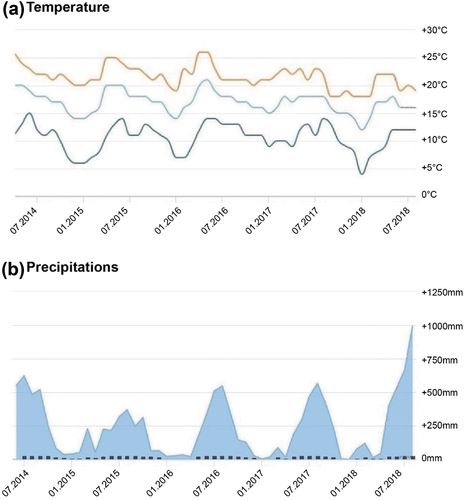
Temperature and humidity records in Nahuatzen over the 2014–2018 period confirmed that most of the annual rainfall occurs during a warm summer rainy season in June–September, while the rest of the year is drier and colder on average (). In August, when salamanders were observed, the air temperature ranged 22.5–25.8°C with a relative humidity of about 66 %.
Figure 3. Climatic records of the Nahuatzen area over the 2014–2018 period. (a) Maximum (red), average (light blue) and minimum (dark blue) temperatures (c); (b) Precipitation (mm, blue areas) and rainy days (black histograms).
Comparisons of water properties with ponds inhabited by other Ambystomatids occurring in adjacent areas (A. flavipiperatum and A. ordinarium), and similarly measured [Citation37], suggested a warmer water, a more basic pH, and a lower conductivity for Nahuatzen, although very few ponds were measured in each case ().
Table 2. Ranges of air temperature and water chemistry in the aquatic sites of A. cf. amblycephalum compared to other Ambystomatids observed during the survey [Citation37]
Genetic assessment
We could sequence, align, and trim portions of the mitochondrial 16S gene (541 bp in final analyses) and the control region (755 bp in final analyses) in one Ambystoma individual from Nahuatzen. For 16S, for which no reference sequence of A. amblycephalum is available, the closest BLAST hit (100% of query cover) was A. mexicanum [99.4% of identity with complete genomes AY659991 and AJ584639). In contrast, the CR sequences received many close hits to various sequences from Citation30, assigned to A. velasci (up to 99.5% of identity], A. ordinarium (up to 99.3% of identity), A. mexicanum (up to 99.2% of identity), A. flavipiperatum (up to 99.1% of identity), A. tigrinum (up to 98.9% of identity) and matched the available A. amblycephalum sequences DQ241132 and U36403 from Iratzio with 98.9% of identity.
Phylogenetic analyses of the concatenated sequences (1,296 bp) supported that the Nahuatzen sample is most closely related to A. mexicanum and A. amblycephalum, but its exact position was not resolved due to poor branch support (). Based on sequences from Citation30, the CR tree was also poorly resolved, and our sample falls into a weakly supported clade including A. velasci (from the central and eastern Mexican Plateau, assigned to A. subsalsum), A. mexicanum and A. amblycephalum ().
Figure 4. A. tigrinum stebbinsi of the Bayesian phylogenetic reconstruction of available Ambystoma mitochondrial sequences (concatenated 16S + control region = 1,296bp). Supported branches (posterior probability >0.95) are indicated by asterisks. The matriline of the Nahuatzen population groups with A. amblycephalum and A. mexicanum.
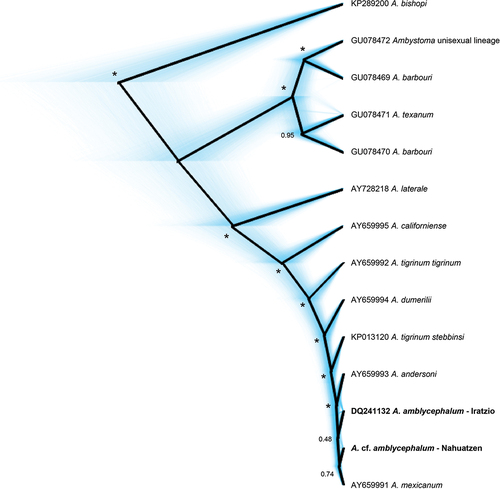
Figure 5. Bayesian phylogeny (maximum clade-credibility tree) of the mitochondrial control region [755bp) from Nahuatzen and other Ambystoma sequences from the study of Citation30. Supported branches (posterior probability >0.95] are indicated by asterisks.
![Figure 5. Bayesian phylogeny (maximum clade-credibility tree) of the mitochondrial control region [755bp) from Nahuatzen and other Ambystoma sequences from the study of Citation30. Supported branches (posterior probability >0.95] are indicated by asterisks.](/cms/asset/b8601fcb-6427-4371-bbfb-dc42bf5c4865/tneo_a_2029323_f0005_b.gif)
Discussion
In this study, we discovered and documented a population putatively assigned to the highly endangered salamander Ambystoma amblycephalum, a species scientifically confirmed only once [Citation17] since its original description 80 years ago [Citation22]. The present locality (Nahuatzen) is situated ~40 km west of both these records and is geographically isolated by Lake Pátzcuaro ().
Morphological and genetic data suggest that the Nahuatzen salamanders are related to A. amblycephalum. First, our metamorphosed individual is morphologically similar to the specimens described by Citation22, and as we summarized above, the other Ambystoma species occurring in Michoacán all have distinctive morphology and coloration. Second, our genetic sample closely matched the A. amblycephalum sequences from Iratzio generated by Citation17 and Citation30. They are not identical, however, but also branch close to lineages documented in A. velasci (central and eastern Mexican Plateau clade), A. flavipiperatum and A. mexicanum (). The complex phylogenetic pattern among this clade, where several taxa are paraphyletic, has been recovered by previous studies [Citation30,Citation38], and could stem from incomplete lineage sorting or recurrent hybridization episodes. Hence, the mitochondrial barcoding should be taken with caution here. Confirming the genetic identity of the new population will require a better phylogenetic resolution, especially using multilocus nuclear markers, which would also allow testing whether the divergence of Nahuatzen compared to Iratzio reflects genetic differentiation due to geographic isolation.
The genetic relatedness between A. amblycephalum and the paraphyletic taxa mentioned above has casted doubt on their species status, especially since they were all described from phenotypic characters [Citation15]. Some of this morphological differentiation could stem from environmental effects [Citation16,Citation17], and we did observe high intraspecific variability in morphology among other Mexican Ambystoma (AH pers. obs.). Accordingly, some authors consider that A. amblycephalum, A. flavipiperatum, the central and eastern Mexican Plateau clade of A. velasci (= A. subsalsum), A. mexicanum, A. andersoni and A. taylori as synonyms [Citation39,Citation40], especially since they belong to the same unresolved clade [Citation30]. New genetic data will be crucial to clarify the systematics of this complex radiation. Here, we follow the present taxonomy where these taxa are listed as a species, and cautiously assign the newly discovered Nahuatzen population as A. cf. amblycephalum, given the imperfect genetic match and potential divergence from the type locality.
Although it frequently appears in some naturalist repositories and museum collections due to misidentifications, A. amblycephalum is critically endangered [Citation23] and even presumed extinct by some long-term monitoring studies [Citation25]. The Nahuatzen population represents the only scientifically confirmed contemporary record attributed to this taxon. Our study also offers new empirical knowledge of its natural history, which can inform future conservation guidelines. In a nutshell, A. cf. amblycephalum requires a temperate subhumid climate (2100–2600 m. a.s.l.) featuring (i) an aquatic habitat consisting of sustainable ponds, small lakes or large reservoirs with water sources, with aquatic plants (reeds, Sypogyra sp.), mud, debris of roots and soils; and (ii) a terrestrial habitat composed of open landscapes scattered by coniferous mixed forests on rich to acidic soils. Areas meeting these criteria should thus be prioritized for future searches, to delimit the (presumably narrow) distribution of this micro-endemic, and quantify its population size. Monitoring of aquatic sites may be possible all year long thanks to the facultative paedomorphy, but tracking terrestrial adults will be challenging given their more cryptic lifestyle, as they putatively remain hidden most of the year in burrows.
At present, the most urgent measure is the protection of the Nahuatzen site, specifically to ensure the maintenance of cattle ponds (or equivalent). Unfortunately, the Purépecha plateau (where Nahuatzen is situated) is currently one of the most threatened forest community of Mexico [Citation41]. The region is been drastically altered by watershed contamination, soil erosion, and deforestation due to illegal logging [Citation42]. Beside the growing human pressure on their habitat, Ambystoma salamanders are also at risk due to predation by introduced fishes (e.g. Cyprinus carpio, Oreochromis aureus and O. mossambicus), poaching by locals, as well as fungal infections [Citation13,Citation23,Citation43].
Nahuatzen’s A. cf. amblycephalum adds to a long list of highly endangered Mexican Ambystoma that are only known from a handful of historical localities, but have (almost) not been seen since their original description many decades ago, e.g. A. bombypellum, A. andersoni, A. dumerilii, A. mexicanum, A. rivulare, A. granulosum, A. flavipiperatum, A. taylori, A. silvense, A. lermaense [Citation9]. The re-discovery of this emblematic amphibian, together with pioneer data on its natural history, thus contributes much-needed hope and insights for amphibian conservation in Mexico.
Author contributions
AH designed the study. AH, JR, EJ, ALS-P, VCR-E and PBN conducted fieldwork. CD and SD performed genetic analyses. AH and CD drafted the manuscript, which was critically improved by all coauthors.
Acknowledgments
We are deeply grateful to David B Wake, Daniel Escoriza, Jérôme Maran, Mian Hou, AmbystoLab and OctoLab for their help and support. We also thank the Secretaria de Medio Ambiente y Recursos Naturales (SEMARNAT) of Mexico for collection permit.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Flores-Villela O. Herpetofauna of Mexico: distribution and endemism. In: Ramamoorthy TP, Bye R, Lot A, et al., editors. Biological diversity of Mexico: origins and distributions. New York: Oxford University Press; 1993. p. 253–280.
- Frías-Álvarez P, Zúñiga-Vega J, Flores-Villela O. A general assessment of the conservation status and decline trends of Mexican amphibians. Biodiver Conserv. 2010;19:3699–3742.
- Stuart SN, Chanson JS, Cox NA, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786.
- Dubois A, Raffaëlli J. A new ergotaxonomy of the order Urodela Duméril, 1805 (Amphibia, Batrachia). Alytes. 2012;28:77–161.
- Duellman WE, Trueb L. Biology of amphibians. New York: McGraw-Hill; 1986.
- Fisher CT, Pollard HP, and Israde-Alcántara I, et al. A reexamination of human-induced environmental change within the Lake Patzcuaro Basin, Michoacan,Mexico. Proc Nat Acad Sci USA. 2003; 100: 4957–4962.
- Ochoa-Ochoa LM, Flores-Villela OA 2016. Ambystoma amblycephalum (ajolote de cabeza chata). In Registros de presencia usados para elaborar el mapa de distribución potencial. Facultad de Ciencias, Universidad Nacional Autónoma de México. Proyecto: JM022, Anfibiofauna endémica frente al cambio climático: análisis de sensibilidad e incertidumbre. Ciudad de México, México: comisión Nacional para el Conocimiento y Uso de la Biodiversidad ( CONABIO).
- Parra-Olea G, Flores-Villela O, Mendoza-Almeralla C. Biodiversidad de anfibios en México. Rev Mex Biodivers. 2014;85:460–466.
- Raffaëlli J. Les Urodèles du Monde. Plumelec, France: Penclen; 2013. Deuxième Édition
- Rovito SM, Parra-Olea G, Recuero E, et al. Diversification and biogeographical history of Neotropical plethodontid salamanders. Zool J Linn Soc. 2015;175:167–188.
- Hernandez A. Etude sur les Urodèles en voie de disparition. Paris: Edilivre; 2016.
- Blackburn DC, and Wake DB. Class Amphibia Gray, 1825. In: Zhang Z-Q editor, Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa. 2011;3148:39–55.
- SEMARNAT. Programa de Acción para la Conservación de las Especies: AMBYSTOMA (Ambystoma sp.). México: SEMARNAT/CONANP; 2018.
- Frost DR. Amphibian species of the world: an online reference. 2021; Available from: https://amphibiansoftheworld.amnh.org/index.php.
- Shaffer HB. Evolution in a paedomorphic lineage. I. An electrophoretic analysis of the Mexican ambystomatid salamanders. Evolution. 1984a;38:1194–1206.
- Shaffer HB. Evolution in a paedomorphic lineage. II. Size and shape in the Mexican ambystomatid salamanders. Evolution. 1984b;38:1194–1206.
- Shaffer HB, McKnight ML. The polytypic species revisited: genetic differentiation and molecular phylogenetics of the Tiger Salamander Ambystoma tigrinum (Amphibia: caudata) complex. Evolution. 1996;50:417–433.
- Contreras V, Martínez-Meyer E, Valiente E, et al. Recent decline and potential distribution in the last remnant area of the microendemic Mexican Axolotl (Ambystoma mexicanum). Biol Conserv. 2009;142:2881–2885.
- Shaffer HB. Natural history, ecology, and evolution of the Mexican “axolotls”. Axolotl Newsletter. 1989;18:5–11.
- Petranka JW. Salamanders of the United States and Canada. Washington and London: Smithsonian Institution Press; 1998.
- Recuero E, Cruzado-Cortes J, Parra-Olea G, et al. Urban aquatic habitats and conservation of highly endangered species: the case of Ambystoma mexicanum (Caudata, Ambystomatidae). Annales Zoologica Fennici. 2010;47:223–238.
- Taylor JD. New salamanders from Mexico, with a discussion of certain known forms. University Kansas Sci Bullet. 1940;26:407–430.
- IUCN SSC Amphibian Specialist Group. 2016. Ambystoma amblycephalum. The IUCN Red List of Threatened Species 2016: e.T59050A53973313
- SEMARNAT. Proyecto de Modificación del Anexo Normativo III, Lista de especies en riesgo de la Norma Oficial Mexicana NOM-059-SEMARNAT-2010, “Protección ambiental-Especies nativas de México de flora y fauna silvestre- Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo”. México City: Diario Oficial de la Federación;2019.
- Huacuz Elías DC 2008. Biología y Conservación del género Ambystoma en Michoacán, México. PhD Dissertation. Mexico: Universidad de Salamanca.
- Brandon RA. Natural history of the axolotl and its relationship to other ambystomatid salamanders. In: editors, Armstrong J, and Malacinski G. Developmental biology of the axolotl. Oxford: Oxford University Press; 1989;13–21.
- Conabio IB-UNAM, Conanp PNUD,INECC. 2020. Reporte de áreas seleccionadas. Explorador de cambio climático y biodiversidad. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Available from: http://www.biodiversidad.gob.mx/pais/cambio_climatico.html.
- Heyer WK, Donnelly MA, McDiamid RW, et al. Measuring and Monitoring Biodiversity, standard methods for amphibians. Washington: Smithsonian institution Press;1994.
- Marx H. Supplementary catalogue of type specimens of reptiles and amphibians in Field Museum of Natural History. Series Fieldiana. Zoology. 1976;69:33–94.
- Weisrock DW, Shaffer HB, Storz BL, et al. Multiple nuclear gene sequences identify phylogenetic species boundaries in the rapidly radiating clade of Mexican ambystomatid salamanders. Mol Ecol. 2006;15:2489–2503.
- Gouy M, Tannier E, Comte N, et al. Seaview Version 5: a multiplatform software for multiple sequence alignment, molecular phylogenetic analyses, and tree reconciliation. Methods Mol Biol. 2021;2231:241–260.
- Bouckaert R, Vaughan TG, Barido-Sottani J, et al. BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Comput Biol. 2019;15:e1006650.
- Darriba D, Taboada GL, Doallo R, et al. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772.
- Rambaut A, Drummond AJ, Xie D, et al. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst Biol. 2018;67:901–904.
- Bouckaert RR. DensiTree: making sense of sets of phylogenetic trees. Bioinformatics. 2010;26: 1372–1273.
- Velazquez A, Toledo V, Luna I. Mexican temperate vegetation. In: Barbour M, Billings W, editors. North American Terrestrial Vegetation. Cambridge, MA: Cambridge University Press; 2000. p. 573–592.
- Hernandez A, Raffaëlli J, Jelsch E, et al. On the verge of extinction in Mexico today: field observations of Ambystoma ordinarium and Ambystoma flavipiperatum with remarks on their habitat and conservation. Herpetol Bull. 2019;147:15–21.
- Wake DB. Declining amphibian populations. Science. 1991;253:860–861.
- Highton R. Detecting cryptic species using allozyme data. In: Bruce RC, Jaeger RG, Houck LD, editors. The biology of plethodontid salamanders. New York: Kluwer Academic/Plenum Publishers; 2000. p. 215–241.
- Molina Sánchez A, Delgado P, González-Rodríguez A, et al. Spatio-temporal approach for identification of critical conservation areas: a case study with two pine species from a threatened temperate forest in Mexico. Biodiver Conserv. 2019;28:1863–1883.
- Takaki TF, Victoria HA, Díaz-Rios R, et al. Tipos de vegetación conforme al sistema INEGI. In: La biodiversidad en Michoacán. Estudio de Estado 2. Vol. I. México: CONABIO; 2019. p. 297–318.
- Gobierno del Estado de Michoacán de Ocampo. 2020. Plan de Desarrollo Integral del Estado de Michoacán 2015-2021. Available at: http://publicadorlaip.michoacan.gob.mx/itdif/2016/71/pladiem_2016-2021.pdf
- Soto-Rojas C, Suazo-Ortuño I. Los ajolotes michoacanos. In: La biodiversidad en Michoacán. Estudio de Estado 2. Vol. II. México: CONABIO; 2019. p. 499–502.