ABSTRACT
High Andean ponds and reservoirs are among the least-studied environments. We evaluated the composition of littoral macrobenthos and how it is affected by the physicochemical conditions of the water in El Pañe reservoir (Peru), located at 4,550 m.a.s.l. Samples were taken between November 2017 and October 2018 from three zones in the reservoir: low (downstream), middle and high (upstream); two of these zones with fish farms (low and middle) and one zone without fish farms (high). The following physicochemical parameters of the water were measured: dissolved oxygen, conductivity, pH and temperature. The macrobenthic community was analysed through diversity indices such as Shannon-Wiener (H’), Simpson’s dominance index (D), Pielou’s evenness (J’), true diversity (D1), and richness (S). The influence of the physicochemical variables on the macrobenthos was estimated by canonical correspondence analysis (CCA) and non-metric multidimensional scaling (NMDS). Dissolved oxygen was found to have lower values (<0.5 mg/l) than specified in the Environmental Quality Standards (EQS). Macroinvertebrate richness for the whole reservoir was 17 families, and the family with highest relative abundance was Chironomidae (42.24% in the low zone, 51% in the middle zone and 40.43% in the high zone). The indices showed greater species richness in the high zone, where there are no fish farms. Dissolved oxygen and conductivity were the main factors determining macrobenthos distribution and composition.
Introduction
High Andean rivers, wetlands and ponds are among the least-studied aquatic ecosystems. It is estimated that 5.24% of tropical South America lies at over 3,000 m above sea level, and the extensive central and northern Andes constitute 94% of the land above this elevation on Earth [Citation1]. Aquatic environments at great altitudes may be affected by organic contamination, agrochemicals, sedimentation and mining waste, and they are particularly threatened by dams and water extraction [Citation1] which makes them extremely susceptible to the effects of global climate change [Citation2,Citation3].
In Peru, many of the high-altitude aquatic ecosystems show deterioration in water quantity and quality, which affects their biological composition [Citation4]. El Pañe Reservoir, located at approximately 4,550 m above sea level, is one of the sources providing water to Arequipa city, after purification [Citation5]. The reservoir is also used for fish farming, which degrades the ecosystem through the addition of large quantities of waste, such as fish excreta and uneaten feed. A significant amount of these nutrients remains dissolved in the water column, fostering eutrophication, with its environmental, economic and social impacts [Citation6].
Because benthic macroinvertebrates may be sensitive or tolerant to different environmental changes in their habitat, which would be reflected in a change in the structure of their communities, using them as bioindicators of water quality has become a useful tool for ongoing comprehensive identification of potential impacts [Citation7–9]. The United Nations (UN), European Union (EU) and RAMSAR Convention suggest the use of benthic macroinvertebrates for assessment of water quality [Citation10,Citation11], and this type of bioassessment is included in legislation on water management in many countries [Citation12,Citation13]. For instance, Europe [Citation14] and Latin America [Citation9,Citation15] have developed and adapted indices based on benthic macroinvertebrate families.
Competent authorities in the management of El Pañe Reservoir, such as AUTODEMA [Citation16] and SEDAPAR [Citation17], have conducted several assessments of water quality employing only physicochemical indicators [Citation16,Citation18]. Therefore, since there is no baseline information on the benthic invertebrate community in the region, assessment of water quality using bioindicators is limited. The purpose of this study was to describe the benthic macroinvertebrate community in El Pañe Reservoir, in areas without and with impact from fish farming and its potential implications regarding water quality. The aims were (1) to characterize the physicochemical conditions of the water in the reservoir; (2) to describe the diversity of benthic macroinvertebrate families; and (3) to determine the influence of the presence of fish farming in the reservoir. The production process of fish farming releases a large amount of residual feed and excreta into the water, which can be oxidized to soluble substances, increasing nitrate and phosphate concentration. Such water contamination affects the macroinvertebrate community, thus providing an indirect measurement of the effects of fish farming [Citation6,Citation19]. This study contributes to advancing the knowledge on high-altitude benthos and is the first study in this field conducted in such an important reservoir of the Arequipa region in Peru.
Materials and methods
Study area
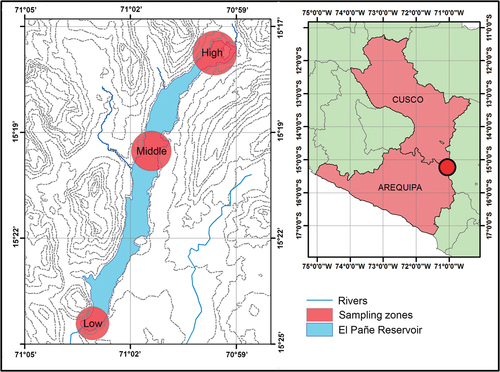
This study was conducted in El Pañe Reservoir, located on the River Negrillo, a tributary of the River Colca, in Caylloma Province, Department of Arequipa (), at an elevation of 4,524 to 4,580 m.a.s.l., in the Altiplano region of southern Peru. The reservoir is part of the headwaters of the River Chili basin. Its active storage capacity is 93 million m3 and dead storage capacity 41.3 million m3. It regulates El Pañe water resource and a wet watershed of 185 km2 [Citation16]. The dam has homogeneous section constituted of fine, silty-clay soil. Its upstream and downstream embankments have a protective rockfill, with a layer of gravels and sands providing a transition between the fine material and the rockfill [Citation16]. The water in the reservoir is used for human consumption in Arequipa city, as well as for irrigation and livestock. The reservoir is used for artisanal Oncorhynchus mykiss (Walbaum, 1792) farming in floating cages. The characteristics of the region are typical of a high Andean pond ecosystem, with mountains and nearby Andean wetlands (“bofedales”). Mean monthly temperature ranges from 6°C in the rainy months (December to March) to 1°C in dry months when cloud cover is lower. Average annual precipitation is 710 mm [Citation20].
Sampling
The sampling sites were located in the low (downstream), middle and high (upstream) zones along the reservoir (). There are fish farms in the low and middle zones but not in the high zone. Ten sampling stations were established: three in the low zone (E1–E3), four in the middle zone (E4–E7) and three in the high zone (E8–E10) (). Six sampling sessions were conducted in November and December 2017 and April, May, July and October 2018. A Hanna HI9829 multiparameter portable meter was used to measure dissolved oxygen (mg/L), pH, conductivity and temperature in the field. During each sampling session, three benthos samples were taken per station: one located randomly plus two repetitions located at least 5 m away (180 samples altogether).
Table 1. Sampling stations at El Pañe Reservoir, Arequipa, Peru
Macroinvertebrates were collected in the benthos over 1 m2 by semi-quantitative kick sampling, using a D-frame dip net (500 µm mesh and 30 cm opening). To take each sample, the substratum was agitated vigorously with the feet for 2 min. The organisms collected during each repetition were stored separately in labelled jars and fixed in 4–5% formalin. In the lab, the samples were preserved in 70% alcohol for identification under stereomicroscope [Citation21,Citation22]. All macroinvertebrate individuals were identified at family level following Domínguez and Fernández [Citation21], Merritt et al. [Citation23], Thorp and Lovell [Citation24] and Prat et al. [Citation25].
Data analysis
The macrobenthos community structure was evaluated using richness (number of families) (S), relative abundance ([number of individuals in a family/total number of individuals in all families]*100), Shannon-Wiener diversity index (H’), Simpson’s dominance index (D), Pielou’s evenness (J’) and true diversity (D1) [Citation26]. A Shapiro–Wilk test was performed to determine whether the indices had a normal distribution. ANOVA was used to compare the community indices in conditions among sampling sites. A standard principal component analysis (PCA) using normalized variance-covariance matrix was performed to determine environmental variability of water quality parameters, and standard canonical correspondence analysis (CCA) was used to evaluate the relationship between the composition of macroinvertebrates and physicochemical variables. The ANOSIM permutation test (with 9,999 replicates) was used to test the hypothesis of differences in the composition of the community between sampling zones and the statistical significance of the groupings. The SIMPER similarity analysis and non-metric multidimensional scaling (NMDS) ordination based on the Bray-Curtis index were used to explore variations in the composition of macroinvertebrate taxa among sampling zones. The analyses were performed using EXCEL® and PAST version 4.03 software [Citation27].
Results
Physicochemical parameters
Surface water temperature, conductivity, pH and dissolved oxygen values are shown in . The conductivity was higher in December in the three sampled zones (low, middle and high). Dissolved oxygen was the only physicochemical parameter that did not meet the Water Quality Standards according to Peruvian legislation (Ministry of Environment [MINAM] Supreme Decree 004–2017), having low values in all three zones in the reservoir (<5 mg/l) ().
Table 2. Mean values and standard deviation of physicochemical parameters in the three sampling zones of El Pañe Reservoir, Arequipa, Peru
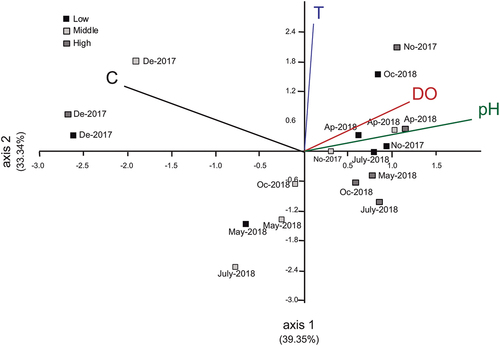
PCA () shows that the first two components explained 72.69% of the total variability between environmental variables. Component 1 had a positive correlation with pH (0.78) and dissolved oxygen (0.52) and a negative correlation with conductivity (−0.83). Component 2 had a positive correlation with temperature (0.96). Samples for December 2018 show high conductivity values.
Figure 2. Plot of zones with sampling months in El Pañe high Andean reservoir in Arequipa, Peru, according to principal component analysis (PCA) for environmental variables: C, conductivity (µS/cm); DO, dissolved oxygen (mg/L); pH; T, temperature (°C). Component 1 explains 39.35% and component 2 explains 33.34% of the total variability.
Diversity of littoral macrobenthos
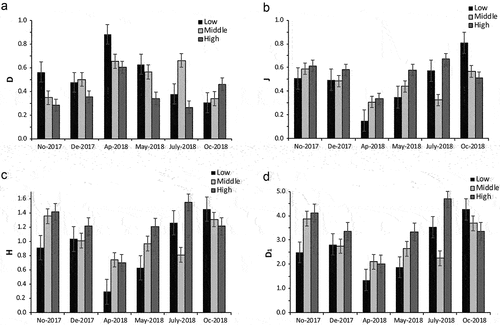
Seventeen macrobenthic families were identified in El Pañe Reservoir. The highest mean relative abundance values were recorded for Chironomidae (). Corixidae followed in abundance in the high zone, Naididae in the middle zone and Lumbriculidae in the lower zone (). The values obtained for community structure indices show that in the low zone (November 2017, April and May 2018) and the middle zone (December 2017, April, May and July 2018), dominance (D) is greater than evenness (J); in contrast to the high zone where there is greater evenness, except in April 2018. Shannon-Wiener diversity index (H) and true diversity (D1) were greater in the high zone than in the low and middle zones, except in April and October 2018 (). The Shapiro–Wilk test showed the normal distribution of the community structure indices (S: W = 0.92, p = 0.319; D: W = 0.91, p = 0.194; H: W = 0.95, p = 0.637; J’: W = 0.97, p = 0.866; D1: W = 0.96; p = 0.802). ANOVA showed that there is no significant difference in D, J’, H and D1 among different zones, while S differed significantly among zones ().
Table 3. Mean relative abundances (%) of aquatic macroinvertebrate families in El Pañe Reservoir, Arequipa, Peru, in each sample zone
Table 4. ANOVA test for community indices among sampling zones. Significant values in bold
Figure 3. Community structure indices for El Pañe Reservoir, Arequipa, Peru, in each sampling session. A, Simpson’s dominance index (D); B, Shannon-Wiener diversity (H); C, Pielou’s evenness (J’); D, true diversity (D1).
The ANOSIM similarity analysis showed that macrobenthos composition in the high zone differed significantly (R = 0.172 and p < 0.05) from that in the low and middle zones. The SIMPER analysis showed that the three zones had a mean dissimilarity of 58.59%. The main families contributing to this dissimilarity were Chironomidae (28.23%), Naididae (20.48%), Corixidae (18.74%) and Lumbriculidae (11.4%).
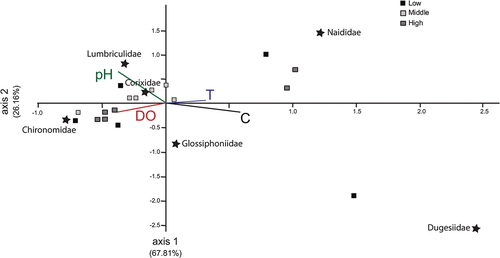
CCA () showed that the accumulated variance of the association between physicochemical variables and community composition was explained by the first two axes in 93.97%. Chironomidae was associated to dissolved oxygen, while Corixidae and Lumbriculidae were associated with pH.
Figure 4. Canonical correspondence analysis (CCA) showing the association among the families present in El Pañe high Andean reservoir in Arequipa, Peru, and the environmental variables considered (vectors). C, conductivity (µS/cm); DO, dissolved oxygen (mg/L); pH; T, temperature (°C).
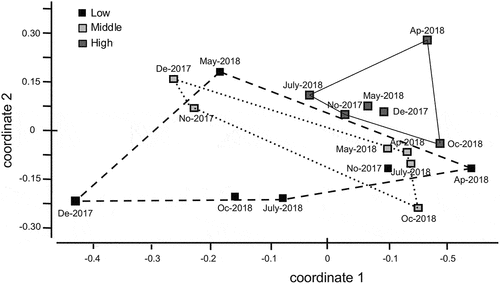
The NMDS analysis provides a graphic representation of the similarity among the zones in the reservoir. The high zone is distinct from the middle and low zones (stress = 0.17; R of axis 1 = 0.47 and R of axis 2 = 0.37) ().
Figure 5. Non-metric multidimensional scaling (NMDS). Samples are distinguished according to zones and months (No, November; De: December; Ap: April; May; July; Oc, October) in the high Andean reservoir El Pañe, Arequipa, Peru. Solid line, samples from high zone; dashed line, from middle zone; doted line, from low zone.
Discussion
The Altiplano region of the Andes is a topographically complex region located at a high elevation, with low atmospheric pressure, high insolation and wide seasonal and daily variations in temperature [Citation28]. Although the aquatic environments in this region are an important resource for the human population, they are among the least studied [Citation28]. Studies on macroinvertebrate richness in rivers in this region have increased in recent years [Citation28–30], but there is limited information for ponds and reservoirs in the Andean Altiplano [Citation1]. This highlights the importance of the findings of the current study, which was conducted in a reservoir at 4,500 m.a.s.l.
The physicochemical characteristics of high Andean rivers are variable and fluctuate over space and time [Citation1]. Comparison of the variables recorded in this study to values published for similar environments shows that the temperature, pH and conductivity ranges are typical of the Andes, due to the geological conditions [Citation29,Citation31,Citation32]. Conductivity found for El Pañe Reservoir in this study was lower than the average reported by Villamarin et al. [Citation29] for the River Colca basin in Perú during the dry season. According to these authors, the high conductivity values in the River Colca, compared to rivers at other latitudes, are due to the basin’s geological characteristics. The current study recorded temporal variation in conductivity. At all sampling sites, conductivity was highest in December, which is the dry season when there is no rainfall and the reservoir is at its lowest level. Dissolved oxygen values were lower than expected for a lentic water body at high altitude [Citation33]. For Lake Titicaca, at 3,810 m.a.s.l., normal dissolved oxygen range is 4.5–7.5 mg/L [Citation33]. This may be explained by the low atmospheric pressure as a result of the elevation of El Pañe Reservoir (4,550 m.a.s.l.) [Citation1]. Also, high altitude streams are close to a critical oxygen level because their natural oxygen saturation at atmosphere equilibrium may be about 60% (e.g. Bolivian Altiplano streams at 3,000 m.a.s.l.). They are thus more sensitive to dissolved oxygen lowering due to organic pollution [Citation28]. The waste from the fish farming load increases the proliferation of microorganisms that break it down, leading to a reduction in dissolved oxygen concentration and an increase in inorganic nutrients such as ammonium and phosphate [Citation34].
Several studies [Citation35–39] report that in lentic systems (regardless of geographic area), the most representative littoral macrobenthos in high-altitude lakes and ponds usually consists of larvae of the family Chironomidae and Oligochaeta at high densities due to the amount of organic matter [Citation40, Citation41] and low oxygen concentrations, because the heme group in their circulatory system enables them to live at low oxygen concentrations. Other studies [Citation42–45] agree in reporting the families Glossiphoniidae, Naididae and Corixidae in ecosystems of this kind. It is important to note that in the high zone in the current study, there is high abundance of the family Corixidae. This may be due to the fact that because there is no fish farm in this zone, there are few free trout, which are the main predators of some aquatic insects [Citation19,Citation44]. Trichoptera and Plecoptera had no representativity, since they usually live under logs, rocks or plant material in fast-flowing, well-oxygenated, cold lotic currents, and these conditions are absent in El Pañe Reservoir.
Species diversity and true diversity were low in all reservoir zones, possibly because there is low diversity of habitats in these ecosystems at high latitudes, and therefore a low supply of ecological niches, conditioning macrobenthos diversity [Citation46]. According to Leiva [Citation45], high diversity values are directly related to the variety of habitats and good balance in communities. Evenness values were low, due to the reduction in abundance of sensitive insects and increase in dominance of families tolerant of hypoxia as a result in the increase in organic matter [Citation47,Citation48], such as Chironomidae and Naididae. This was noted mainly in the low and middle zones, where there are fish farms. NMDS differentiated the high zone without impact from fish farming and grouped the two zones impacted by fish farming (low and middle zones), reflecting the fact that benthic macrofauna is a good indicator of this condition.
Numerous studies highlight the importance of the impact of pH, conductivity, dissolved oxygen, and total suspended solids on the distribution of benthic macrofauna in lotic systems [Citation21,Citation22,Citation49–51], though there is limited available information of this kind for lentic environments and even less for high Andean lentic environments. The CCA confirmed the importance of dissolved oxygen in high Andean ecosystems, where it was found to be associated to families tolerant to low levels of dissolved oxygen such as Chironomidae. In the current study, the family Naididae was associated with conductivity. With regard to other representative families, such as Dugesiidae and Glossiphoniidae, their trophic functionality may be more relevant than the influence of the physicochemical variables studied.
The results of this study show differences in the representativity of the benthic macroinvertebrates in high Andean zones with and without fish farms. It has been shown that intensive fish farming produces ecological effects at multiple levels in high Andean environments [Citation19], in addition to the effects of the accumulation of organic matter at the bottom, dead organic matter and the proportion of uneaten food [Citation6].
Authors’ participation
PCP, AACHD, CVMS, CECR and EWCR conducted the fieldwork and analysis and wrote the manuscript. CD did the final review of the paper.
Geolocation information
Samples from El Pañe Reservoir, Arequipa, Peru, between −15.297076° to −15.419600° latitude, and −71.006565° to −71.067002° longitude.
Acknowledgments
We thank Universidad Nacional de San Agustín for financing the study through UNSAfunds, Contract IBA-0010-2017-UNSA, and the anonymous reviewers for their valuable comments that substantially improved the quality of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Jacobsen D. Tropical high-altitude streams. In: Dudgeon D, editor. Tropical Stream Ecology. London: Elsevier Sience; 2008. p. 219–256.
- McGregor G, Petos PE, Gurnell AM, et al. Sensitivity of Alpine stream ecosystems to climatic change and human impacts. Aquat Conserv. 1995;5(3):233–247.
- Báez S, Jaramillo L, Cuesta F, et al. Effects of climate change on Andean biodiversity: a synthesis of studies published until 2015. Neotrop Biodivers. 2016;2:181–194.
- Samanez I, López D. Distribución geográfica de Boeckella y Neoboeckella (Calanoida: centropagidae) en el Perú. Revista Peruana de Biología. 2014;21(3):223–228.
- Servicio de Agua Potable y Alcantarillado de Arequipa. Alternativas de Solución a la Problemática de la Calidad Hidrobiológica del Agua para el Consumo Humano en la Provincia de Arequipa. Informe Técnico. Mesa Regional de Trabajo para abordar la Problemática del Abastecimiento y Agua Potable para Consumo Humano en la Provincia de Arequipa. 2015.
- Buschmann AH. Impacto ambiental de la acuicultura. El estado de la investigación en Chile y el Mundo. In: Registro de Problemas Públicos. Vol. 4. Santiago, Chile: Terram Publicaciones; 2001. p. 67.
- Castellanos Romero K, Del Río J P, Cuentas Villareal K, et al. Lentic water quality characterization using macroinvertebrates as bioindicators: an adapted BMWP index. Ecol Indic. 2017;72:53–66.
- Roldán-Pérez G, Ramírez Restrepo JJ. Fundamentos de Limnología Neotropical. 2da edición ed. Medellín Colombia: Universidad de Antioquia; 2008.
- Roldán-Pérez G. Los macroinvertebrados como bioindicadores de la calidad del agua: cuatro décadas de desarrollo en Colombia y Latinoamerica. Rev Acad Colomb Cienc Ex Fis Nat. 2016;40(155):254–274.
- de la Convención de Ramsar S. Inventario, evaluación y monitoreo: marco Integrado para el inventario, la evaluación y el monitoreo de humedales. Manuales Ramsar para el uso racional de los humedales, 4ª edición,Vol. 13.Secretaría de la Convención de Ramsar, Gland (Suiza); 2010. https://www.ramsar.org/sites/default/files/documents/pdf/lib/hbk4-13sp.pdf
- Directiva 2000/60/CE del Parlamento Europeo y del Consejo de 23 de octubre de 2000 por la que se establece un marco comunitario de actuación en la política de aguas; 2000.
- Moya N, Hughes RM, Dominguez E, et al. Macroinvertebrate-based multimetric predictive models for evaluating the human impact on biotic condition of Bolivian streams. Ecol Indic. 2011;11:840–847.
- Pond GJ, Bailey JE, Lowman BM, et al. Calibration and validation of a regionally and seasonally stratified macroinvertebrate index for West Virginia wadeable streams. Environ Monit Assess. 2013;185:1515–1540.
- Sandin L, Hering D. Comparing macroinvertebrate indices to detect organic pollution across Europe: a contribution to the EC water framework directive intercalibration. Hydrobiologia. 2004;516(1–3):55–68.
- Ríos-Touma B, Acosta R, Prat N. The Andean Biotic Index (ABI): revised tolerance to pollution values for macroinvertebrate families and index performance. Rev Biol Trop. 2014;62(Suppl. 2):249–273.
- Autoridad Autónoma de Majes. Monitoreo de calidad de agua. Reporte hidrobiológico y fisicoquímico. (enero-Julio). Gobierno Regional de Arequipa; 2017. https://www.autodema.gob.pe/monitoreo-de-la-calidad-de-agua-en-los-embalses/
- SEDAPAR. Informe Técnico “Alternativas de Solución a la Problemática de la Calidad Hidrobiológica del Agua para el Consumo Humano en la Provincia de Arequipa”. Mesa Regional de Trabajo para abordar la Problemática del Abastecimiento y Agua Potable para Consumo Humano en la Provincia de Arequipa. 2015.
- Autoridad Nacional del Agua. Evaluación integral de la calidad del agua de los embalses y ríos que conforman el sistema hidráulico Chili-Arequipa. Informe Técnico 21; 2014. www.repositorio.ana.gob.pe/handle/20.5000.12543/2051
- Moulliet C, Barta B, Espinosa R, et al. Ecological effects of introduced rainbow trout (Oncorhynchus mykiss) in pristine Ecuadorian high Andean lakes. Fundam Appl Limnol. 2018;191(4):323–337.
- ANA. Autoridad Nacional del Agua. Proyecto de Modernización de la Gestión de los Recursos Hídricos. Diagnosticos de problemas y conflictos en la gestión del agua en la Cuenca Chili-Qulica. Lima Peru; 2008. https://repositorio.ana.gob.pe/handle/20.500.12543/2044
- Domínguez E, Fernández H. Macroinvertebrados bentónicos sudamericanos. Sistemática y Biología. Argentina. Tucumán:Fundación Miguel Lillo Tucumán;2009. p. 635–639.
- Roldán G. Bioindicación de la calidad del agua en Colombia: propuesta para el uso del método BMWP. Antioquía: Universidad de Antioquia; 2003.
- Merritt RW, Cummins KW, Berg MB. An introduction to the aquatic insects of North America. Fourth. Dubuque: Kendall/Hunt Publishing Company; 2008. 1158. 10.1899/28.1BR.266.1
- Thorp J, Lovell LL. Phylum Annnelida. In: Thorp J, Rogers C, editors. Covich’s Freshwater Invertebrates. Vol. II. Key to Neartic Fauna. London: Elsevier; 2015. p. 223–263.
- Prat N, Rieradevall M, Acosta R, et al. Guía para el reconocimiento de larvas de Chironomidae (Díptera) de los ríos altoandinos de Ecuador y Perú. Barcelona España: Grupo de Investigación FEM; 2011. p. 41.
- Moreno CE. Métodos para medir la biodiversidad. M&T Manuales y Tesis Sociedad Entomológica Aragonesa. 2001;1(1):1–84.
- Hammer O, Harper DAT, Ryan PD. PAST: paleontological Statistics software for education and data analysis. Paleontol Electronica. 2001;4(1):1–9.
- Jacobsen D, Marín R. Bolivian Altiplano streams with low richness of macroinvertebrates and large diel fluctuations in temperature and dissolved oxygen. Aquat Ecol. 2008;42:643–656.
- Villamarín C, Prat N, Rieradevall M. Caracterización física, química e hidromorfológica de los ríos altoandinos tropicales de Ecuador y Perú. Lat Am J Aquat Res. 2014;42(5):1072–1086.
- Villamarín C, Rieradevall M, Prat N. Macroinvertebrate diversity patterns in tropical highland Andean rivers. Limnetica. 2020;39(2):677–691.
- Guyot JL. Evolución en el espacio y tiempo de las concentraciones de materia en solución y en suspensión, de las aguas de la cuenca Amazónica de Bolivia. In: En DC, editor. Primer symposium de la investigación francesa en Bolivia. La Paz. Bolivia: Embajada de Francia, Ministerio de Planeamiento, Academia Nacional de Ciencias; 1986. p. 48–53.
- Iltis A. Datos sobre las lagunas de altura de la región de La Paz. La Paz: UMSA, ORSTON; 1988.
- Vásquez W, Talavera M, Inga M. Evaluación del impacto en la calidad de agua debido a la producción semi intensiva de trucha (Oncorhynchus mykiss) en jaulas flotantes en la laguna Arapa - Puno. Revista de la Sociedad Química del Perú. 2016;82(1):15–28.
- Gil J. Determinación de la calidad del agua mediante variables fisicoquímicas, y la comunidad de macroinvertebrados como bioindicadores de calidad del agua en la cuenca del río Garagoa Tesis. Universidad de maizales Facultad de ciencias contables económicas y administrativas. 2014.
- Lenihan H, Micheli F. Soft-sediment communities. In: Bertness M, Gaines S, Hay M, editors. Marine community ecology. Sunderland MA US: Sinauer Associates, Inc; 2001. p. 253–287.
- Alonso A, Camargo JA. Estado actual y perspectivas en el empleo de la comunidad de macroinvertebrados bentónicos como indicadora del estado ecológico de los ecosistemas fluviales españoles. Ecosistemas. 2005;14(3):1–12.
- Prat N, Rieradeval M. Criterios de evaluación de la calidad del agua en lagos y embalses basados en los macroinvertebrados bentónicos. Actualidades Biológicas. 1998;20(69):137–147.
- Polo C. Composición y abundancia de los invertebrados bentónicos de Caño Neima, Guajira Venezolana, Estado Zulia. Trabajo Especial de Grado, Departamento de Biología, Facultad Experimental de Ciencias, Universidad de Zulia. Maracaibo. 2005;110.
- Cavero JG, Carhuas M, Mary F. Lagunas altoandinas de Huánuco evaluadas para el desarrollo de acuicultura. Inf Inst Mar Perú. 2019;46(2):236–268.
- Epler JH. Identification manual for the larval Chironomidae (Diptera) of North and South Carolina. North Carolina, North Carolina Department of Environmental and Natural Resources – Division of Water Quality. 2001.
- Caleño R, Rivera C, Ovalle H. Hábitos alimentarios de quironómidos (Diptera: chironomidae) en lagos del páramo de Chingaza, Colombia. Rev Biol Trop. 2018;66(1):136–148.
- Mariano M, Huaman P, Mayta E, et al. Contaminación producida por piscicultura intensiva en lagunas andinas de Junín, Perú. Rev Perú Biol. 2010;17(1):137–140.
- González ER, Watling L. A new species of hyalella from the Andes in Perú (Crustacea: amphipoda: hyalellidae). Re biol trop. 2002;50(2):649–658.
- Contreras G, Navarrete N, Fernández E, et al. Aspectos ecológicos de los Corixidae (Hemiptera, Heteroptera) en el estanque piscícola “GL” de Soyaniquilpan de Juárez, Estado de México. Hidrobiológica. 2001;11(1):53–60.
- Leiva M. Macroinvertebrados bentónicos como bioindicadores de calidad de agua en la cuenca del estero Peu Peu comuna de Lautaro IX Región de la Araucania. Tesis. Facultad de Ciencias de la Universidad Católica de Temuco, Araucanía. 2004.
- Acosta R. Estudio de la cuenca altoandina del río Cañete (Perú): distribución altitudinal de la comunidad de macroinvertebrados bentónicos y caracterización hidroquímica de sus cabeceras cársticas. Memoria de título de Doctor por la Universidad de Barcelona. 2009.
- Melcalfe J. Biological water quality assessment of running waters based on macroinvertebrate communities: history and present status in Europe. Environ Pollut. 1989;1989(1–2):101–139.
- Herbas R, Rivero F, Gonzales A. Indicadores biológicos de calidad del agua Cochabamba. Universidad Mayor de San Simón, Facultad de Ciencias y Tecnología. 2006.
- Mancilla G, Valdovinos M, Azocar M, et al. Efecto del reemplazo de la vegetación nativa de ribera sobre la comunidad de macroinvertebrados bentónicos en arroyos de climas templados, Chile central. Hidrobiología. 2009;19:193–203.
- Nieves G, Rosas Rodríguez RK, and Devaire Hornedo ME. Biodiversidad de insectos acuáticos asociados a la cuenca del río Grande de Manatí. Edición revisada. Departamento de Recursos Naturales y Ambientales. San Juan Puerto Rico: Puerto Rico; 2010. http://www.drna.gobierno.pr/oficinas/saux/secretaria-auxiliar-de-planificacion-integral/planagua/proyecto-rios-patrimoniales/estudio-debiodiversidad-de-insectos-acuaticos.pdf
- Soldner M, Stephen I, and Ramos L, et al. Relationship between macroinvertebrate fauna and environmental variables in small streams of the Dominican Republic. Water Res. 2004;38(4):863–874.