ABSTRACT
Breeding in colonies is a defining characteristic of many waterbird species and several behavioural and habitat traits determine nest-site selection. Although there is relatively extensive literature on the topic, information on the within-colony distribution of nests lacks for mixed colonies, especially for ardeid-cormorant associations and in regions such as Central America. Here, geospatial data on vegetation associated with a mixed colony, established in a small alluvial forest northwest of El Salvador and collected from June to November 2015 was assessed to identify some behavioural and habitat features that could influence nest-site selection. Four ardeid species (Ardea alba, Egretta thula, Bubulcus ibis, and Nycticorax nycticorax) and one cormorant (Phalacrocorax brasilianus) conformed the colony, where the last was the outstanding species. The total area of the colony was 2.14 ha (14% of La Barra forest) with high nest densities (584.58 nests/ha). Spatial analyses indicate that some species prefer to occupy trees/shrubs close to other trees/shrubs with conspecific or similarly sized species. A remarkable height-strata distribution was found, with large-sized species in the canopy, and small-sized species in the lower strata. The resulting estimates of the generalized linear models applied suggest that the within-colony nest-site selection is determined by the combination of higher heights of the tree/shrub used for nesting, nearest distance to a food source, fewer species sharing the same tree/shrub and an increasing effect of the interaction of the latter two factors. Finally, the temporal evolution of La Barra’s colony shows that the increasing number of Neotropical Cormorants (Phalacrocorax brasilianus) could be shaping and threatening the colony’s species structure and possibly the vegetation as well. Conservation measures should be implemented to manage the population of this species.
Introduction
Concentrating in colonies is a defining characteristic of many waterbirds and generally, these species congregate to nest as well [Citation1]. In mixed-species nesting colonies, great numbers of breeding birds coexist in a site, leading to dense nested zones and partitioning of the available area [Citation1,Citation2]. Colony location is species-specific and is usually determined by the proximity of food sources, nest-site availability, vegetation structure, and hydrological regimes in the wetland [Citation2–5]. Within the colony, the nest-site selection is related to the quality of the habitat and predation [Citation6]. In homogeneous habitats (i.e. in terms of physical quality, nest-sites available are relatively equal throughout the habitat), the breeding pairs tend to nest at the centre of the colony as it offers the highest benefits in terms of group protection and vigilance, and, therefore, a central-periphery nest density gradient would be expected [Citation6]. Conversely, in heterogeneous habitats, nest-site selection is based on the most suitable and high-quality nesting sites available, which do not necessarily correspond to a central-periphery gradient [Citation7]. In this case, pairs may trade central nesting locations, which offer better predator protection but are of lower quality, with high-quality sites at the peripheries of the colony that assures the nest success [Citation6–8].
A high-quality nesting site is attributable to the vegetation structure, which in the case of heterogeneous habitats, permits a wide space of nest-site availability in terms of vegetation height [Citation9,Citation10]. The vertical composition of the vegetation is a key trait that determines the species stratification of a tree-nesting waterbird colony [Citation11]. Previous studies suggest that in mixed and non-mixed colonies, this vertical composition is related to species-specific traits, such as body mass, age of the breeding pairs, and the arrival timing for nesting [Citation12–14]. Thus, higher-quality pairs, that is, more experienced and/or larger individuals, will occupy the best and highest nest-sites early in the reproductive season, while the low-quality pairs are forced to nest at the lowest and peripheral positions. In mixed colonies, the same pattern occurs although adding a vertical stratification by species, with the larger ones nesting at the upper stratum and those of smaller size on the lower [Citation12–14]. The intra- and interspecific social interactions is another feature that determines the within-colony nest distribution [Citation15,Citation16]. There is evidence that, in mixed colonies, pairs prefer to nest close to a conspecific or with the species most similar in size as a way to avoid interspecific competition against larger species [Citation9,Citation12,Citation14–16].
Although there is a relatively extensive literature on the within-colony nest distribution of waterbirds, information of breeding colonies at Neotropical habitats such as those in Central America is scarce, as well as little is known about how this nest-site selection in mixed colonies is determined by the combination of several factors such as food resources, neighbouring nest preference, and vegetation. Here, a mixed-species colony established in La Barra, a small-sized alluvial forest in the northwest of El Salvador, was studied using geospatial data, height measures of the vegetation, and the number of nests per breeding species during the reproductive season of 2015. In 2000, a breeding colony of Great Egrets (Ardea alba; Linnaeus, 1758) and Cattle Egrets (Bubulcus ibis; Linnaeus, 1758) was documented for the first time in La Barra forest [Citation17]. Since then, the size of the colony has increased, and new species of waterbirds have established [Citation17,Citation18]. To date, Great Egrets, Cattle Egrets, Neotropical Cormorants (Phalacrocorax brasilianus; Gmelin 1789), Snowy Egrets (Egretta thula; Molina 1782), Black-crowned Night Herons (Nycticorax nycticorax; Linnaeus, 1758), Bare-throated Tiger Heron (Tigrisoma mexicanum; Swainson, 1827),) and Tricolored Herons (Egretta tricolor; Statius Müller, 1776), are known to have nested at least once at the site [Citation18,Citation19]. According to Herrera et al. [Citation18] and Ibarra et al. [Citation19] the reproductive season at La Barra forest starts in February with Great Egrets, showing a nesting peak between April and June and ends in September with Neotropical Cormorants.
La Barra forest is an unusual site and of conservation concern, since it is the only reported colony in the north of the country, and presents a small-size area with several associated anthropogenic threats such as illegal logging, soil extraction, and waterbird poisoning by pesticides, although still suitable for the annual nesting of these species [Citation19]. The present study aims to (a) provide an assessment of the state of the colony based in the number of nests, the actual expansion of the colony and vegetation structure and occupancy, (b) analyse the within-colony horizontal and vertical distribution of the nests per species, (c) and determine the factors influencing the within-colony nest-site selection based on the vegetation occupancy for nesting.
Materials and methods
Study site and data collection
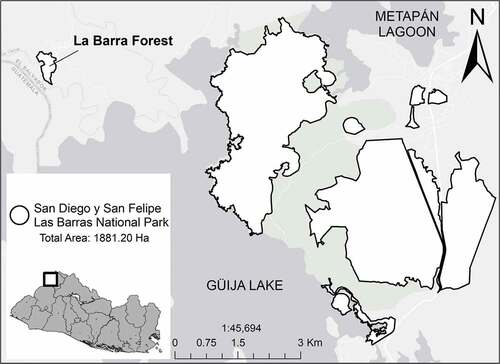
The La Barra forest is located in La Barra village, in the municipality of Metapán, department of Santa Ana (El Salvador) (14°17’ N, 89°28’ W) (). The altitudinal range is 410–420 m a.s.l., and has an area of 15.17 ha of Alluvial Forest and an Evergreen Forest predominant habitat [Citation20–22]. The La Barra forest is constituted of three layers: A first canopy with trees up to 40 m high, with Ceiba pentandra (L.) Gaertner, Sloanea tenuiflora (Mociño & Sesse) Standley, Terminalia oblonga (Ruiz & Pav.) Steud., and Brosimum alicastrum Sw. as the predominant species; a second canopy of 10–20 m, with species such as Triplaris melaenodendron (Bertol.) Standley & Steyerm., Coccoloba caracasana Meissner, Tabebuia rosea (Bertol.) Bertero ex A.DC., and Albizia caribae (Urb.) Britton & Rose; and a herbaceous layer with a predominance of Heliconia sp [Citation20]. During the wet season, the Ostúa River floods the La Barra forest, which temporarily forms natural water channels due to the poor drainage of the silty-type azonal soils that characterize this forest [Citation22,Citation23].
Figure 1. Map of the San Diego and San Felipe Las Barras National Park showing the La Barra forest location.
The sampled area was the entire La Barra forest [Citation24], and data was collected over 12 days distributed between the months of June and November in 2015. To determine the maximum size and phenology of the colony, all active nests were counted in the months of June (fourth week), July (second week), August (first and fourth week), and September (fourth week) [Citation25]. Trails were walked through, and around the colony [Citation26,Citation27] and Bushnell 7–15 × 25 binoculars and bird identification guides [Citation23,Citation28] were used to facilitate species identification and observation. Each plant form (shrubs and trees) used for nesting was georeferenced with a Garmin GPSmap 62 portable device (error = 3 m), and identified by marking the trunk with bright orange spray paint with an alphanumeric code and all active nests were counted for each tree/shrub. During the weeks with the highest density of active nests, each nest-count was carried out simultaneously by two observers and by consensus; the number of nests per tree/shrub was estimated [Citation27]. The height of each previously marked tree/shrub was measured using a Fluke 414D laser distance meter (50 m) and following Larjavaara and Muller-Landau [Citation29]. These measures were made in days with less nest densities (August). To decrease biases in height estimations, distance measures were done 2–3 times per tree/shrub.
Descriptive
Nesting area and densities
The total area of the colony was calculated using ArcGIS 10.3, by creating the Minimum Convex Polygon (MCP). The MCP is a simple tool that allows measuring the total extension area of a number of feature points and where none of their internal angles exceed 180° [Citation30]. Using the MCP and the maximum number of nests recorded, the nesting density per bird species was calculated as Da = number of nests i/MCP. Likewise, the nest density of each bird species per plant form was calculated as Dp = number of nests i/number of trees/shrubs. Values are shown to be determined by the mean ± standard error. The Kruskal–Wallis test was used to determine whether there were significant differences between nesting species densities per plant form (Dp) and was performed in R [Citation31]. Dp was projected in ArcGIS 10.3 using the Kernel density tool and applying the Silverman’s Rule of Thumb for estimating the smoother parameter [Citation32], since there are no available values for nesting colony’s areas in the literature and also because it is robust to spatial outliers.
Colony’s spatial distribution
To analyse whether the within-colony spatial distribution of each species is dependent on the species established (whether con- or heterospecific) in the closest tree/shrub, the X2 test of independence was used and performed in R [Citation31]. To assess the significant contribution of each category of the X2 test, Agresti’s [Citation33] approach was followed, for which an absolute value of Pearson’s standardized (adjusted) residual >2.0 indicates lack of fit of the null hypothesis and hence a strong contribution of the category to the test significance. More specifically, a standardized residual value >2.0 indicates that the number of cases in that cell is significantly higher than would be expected if the null hypothesis were true, with a significance level of p = 0.05. If this value is < −2.0 it indicates that the number of cases in that cell is significantly less than expected if the null hypothesis were true [Citation33].
Colony’s vertical distribution
To analyse whether the nesting birds were vertically distributed according to a height stratum, each species was categorized into three altitudinal levels based on the canopy layers of La Barra forest [Citation20]: low (1 to 10 m), medium (10.1 to 20 m), and high (20.1 to >30 m). To create these categories, the height of the trees/shrubs where the birds established their nests was used. The X2 test of independence was used to compare both nominal variables of bird species vs height strata. To assess the significant contribution of each category of the X2 test, Agresti’s [Citation33] approach was followed again. Cramer’s V coefficient was used to assess the degree of relationship between both variables using the vcd (visualizing categorical data) package [Citation34].
Factors influencing the within-colony distribution
To analyse the factors that influence the selection of the within-colony distribution of waterbirds in the La Barra forest, generalized linear models (GLMs) were used. The response variable was the number of nests per plant individual since a high concentration of nests is a good indicator of a suitable site for nesting [Citation7,Citation8]. Due to the nature of the variable (count data), the Poisson distribution and log link function were used and scaled for extra-variation (quasi-Poisson regression) [Citation35]. The explanatory variables chosen were: height of the plant species (Height, numerical), nearest distance to the closest urban area (Urban, numerical), nearest distance to the closest body of water (River, numerical), nearest distance to the closest tree/shrub within the colony (Neighbour), the tree/shrub taxonomic family (Family, categorical, 15 levels), and the number of bird species that share the same tree/shrub (Sharing, categorical, 3 levels). The closest urban area found was La Barra urbanization to the east of the colony, and at ~76 m, and the closest body of water was the Ostúa River, to the west and at ~270 m from the nesting colony. The nearest distances measurements (Urban, River, and Neighbour plant) were made on ArcGIS 10.3, using the geolocation of each tree/shrub occupied by nesting and the closest distance to the feature to be analysed. Data exploration was based on Zuur et al. [Citation36] and consisted of the observation of outliers (boxplots), normality (histogram), and bivariate relationships (Spearman’s rank correlation test for non-normally distributed variables) between covariates and response variables. Numerical variables were log-transformed to achieve normality. To deal with the collinearity between both categorical and numerical explanatory variables, the generalized variance-inflation factor (GVIF) was inspected [Citation36]. Height, River, Neighbour, and Sharing had GVIF values below 1.5 and were used as explanatory variables in the GLMs, modelled as additive effects and specific interaction terms (Sharing*Height, Sharing*River, and Sharing*Neighbour). We use F-distribution approximations to the deviance ratios to calculate the significance of the effects. The model validation was assessed using graphical tools [Citation36].
Results
Descriptive data of the waterbird colony and associated vegetation
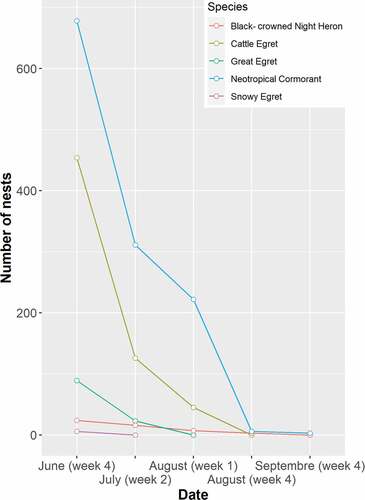
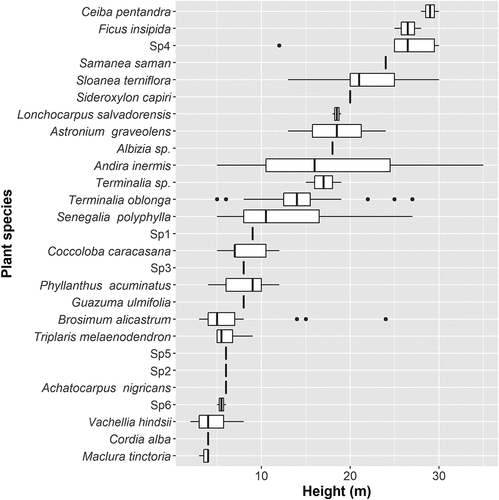
During the last week of June, the highest number of active nests in the colony was 1251 (). The colony consisted of five species: Great Egret, Cattle Egret, Snowy Egret, Black-crowned Night Heron, and Neotropical cormorant. The Neotropical Cormorant and the Cattle Egret had the highest nest abundance (54% and 36%, respectively) (). About 172 shrubs and trees of 28 species were occupied for nesting, of which 22 were identified as genus, and 20 as species, corresponding to a total of 14 families. Six species were not identified. The plant species with the highest frequency of occupation by the nesting colony were: Senegalia polyphylla (DC.) Britton & Killip (19%), B. alicastrum (13%), T. oblonga (11%), Andira inermis (W.Wright) DC (11%), and Vachellia hindsii (Benth.) Seigler & Ebinger (11%). The remaining 35% corresponds to 18 species with frequencies <10. Senegalia polyphylla and Sp4 were used by all the nesting species (). Ceiba pentandra was the tree species with the highest mean height (mean = 29 ± standard error = 1 m), followed by Ficus insipida Willd. (26.5 ± 1.5 m), and Sp4 (25 ± 2.75) ( and ).
Table 1. Maximum nest numbers of the waterbird colony in La Barra forest during the reproductive season of 2015 in San Diego and San Felipe Las Barras National Park
Nesting area and densities
The total area of the nesting colony was 2.14 ha, approximately, 14% of La Barra forest, and a total nest density of 584.58 nests/ha (). At the species level, the Neotropical Cormorant and the Cattle Egret showed the highest densities in the colony with 316.85 nests/ha and 212.17 nests/ha, respectively. The total nest density per plant was 7.28 ± 7.97. Significant differences were found between the species (Kruskal–Wallis = 88.10, df = 4, p < 0.001), were the Neotropical Cormorant and the Cattle Egret showed the highest values with 11.49 ± 1.41 nests/tree and 4.68 ± 0.23 nests/tree, respectively ().
Colony’s spatial distribution
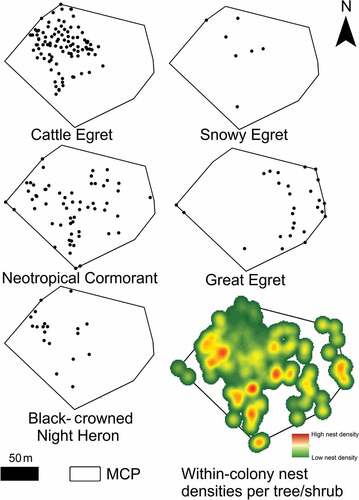
Nest site distribution per species shows that Cattle Egrets form a nucleus in the northwest of the colony, while Great Egrets establish their nests in the periphery, the Snowy Egret and the Black-crowned Night Heron mix with Cattle Egrets, and the Neotropical Cormorant in a more random distribution (). The closest neighbouring species analysis showed that species tended to nest in trees/shrubs close to conspecifics or species of similar size (X2 = 140.79, df = 16, p < 0.01). For Black-crowned Night Herons, 71.4% of the trees/shrubs used for nesting were close to Cattle Egrets (residuals = 2.1). Snowy Egrets tend to nest in trees/shrubs close to Cattle Egrets as well (71.4%), although low residual values due to small sample size contribute little to the significance of the test (1.2). On the contrary, Cattle Egrets tended to establish their nests mainly in trees/shrubs close to conspecifics (66.9%, residuals = 5.4), and a little percent close to Black-crowned Night Herons (21.8%, residuals = 3.2). Great Egrets showed a strong preference to nest in trees/shrubs close to conspecifics (55.6%, residuals = 7.9), and 40.7% to Neotropical Cormorants (residuals = 2.8). Neotropical Cormorants tended to nest in trees/shrubs close to conspecifics as well (40.7%, residuals = 4.5) and a minor percentage to Great Egrets (18.6%, residuals = 2.2).
Colony’s vertical distribution
The nesting colony showed a vertical distribution according to a height stratum (X2 = 179.56, df = 8, p < 0.01) (). Both variables showed a high correlation (Cramer’s V = 0.61, p < 0.01). Under the premise that the categories with absolute values of Pearson residuals >2 strongly contribute to the significance of the X2 test, the Great Egret is considered as a bird that nests mostly in the highest layer of the forest (55.6%), the Neotropical Cormorant in the middle and high strata (64.4% and 32.2%, respectively) and the Cattle Egret in the lower stratum (91.1%). The Snowy Egret and the Black-crowned Night Heron nested mostly in the lower stratum. However, the contribution to the significance of the X2 test was low due to the small number of nests present at the colony, and therefore unreliable (Pearson residuals <2).
Table 2. Basic descriptive statistics of the plant species used by the nesting colony during the reproductive season of 2015 in La Barra forest, San Diego and San Felipe Las Barras National Park
Table 3. Vertical distribution of nesting waterbirds in La Barra forest
Factors influencing the within-colony distribution
Low but significant correlations were found between the number of nests per plant individual and Height (Spearman = 0.26, p < 0.01), and between Height and River (Spearman = 0.21, p < 0.01). At species level, several bivariate correlations were also found for Great Egrets, Height and River (Spearman = −0.69, p < 0.01) and Height and Urban (Spearman = 0.69, p < 0.01); for Neotropical Cormorant, number of nests and Height (Spearman = 0.36, p < 0.01); for Black-crowned Night Herons, number of nests and Height (Spearman = 0.49, p = 0.02) and Height and River (Spearman = 0.50, p = 0.02); and for Cattle Egret, number of nests and Height (Spearman = 0.50, p < 0.01), number of nests and River (Spearman = −0.18, p = 0.04), Height and River (Spearman = 0.24, p < 0.01), and Height and Urban (Spearman = −0.25, p < 0.01).
The best quasi-Poisson regression model shows that the number of nests per plant is determined by Height, River, Sharing, and the interaction of River and Sharing. All variables were significant in this model (p < 0.05) with the exception of one category on the Sharing variable (the one accounting for three species per tree/shrub, p = 0.57). The non-significance of the category is given by the low counting frequency (n = 1) and is also reflected in the non-determined coefficient of the second River*Sharing interaction term (). Recoding the variable in fewer dummy categories was tried to avoid this problem, however, doing this increased the overdispersion of the model, so the four-category option was chosen at the cost of losing this estimate. However, the identifiability of the other categories is sufficient to support the biological meaning of the Sharing variable. The mean Height of plants was 12.25 m, which varied from 2 to 35 m and the log-transformed estimate indicates that higher nest densities per plant will increase as the tree/bush height increases. For the River variable, the mean nearest distance is 348.10 m, varying from 265.6 to 449.6 m, and the log-transformed estimate indicates that at shorter distances to Ostúa River, there is a greater probability of finding more nest densities. The Sharing variable estimates indicated that highest nests densities were explained by fewer species sharing the same tree/bush, where about 81% of the trees/shrubs were occupied by one species, 18% by two species, and 1% by three and four species. The River and Sharing interaction terms indicates that the increase of both factor interactions is also determining the increment of nest densities in trees/shrubs ().
Table 4. Variables related to the within-colony nesting selection site in La Barra forest during the reproductive season of 2015
Discussion
Breeding species, timing, and vegetation associated
The nesting species and the nesting timing registered here coincide with previous studies in the La Barra forest [Citation18,Citation19], although the nest numbers do not. As in here, Herrera et al. [Citation18] described the reproductive phenology of the colony from April to May, decreasing in June and ending the season with the Neotropical Cormorant in September. At a species level, differences in the reproductive phenology were found with Ibarra-Portillo et al. [Citation19]. For example, according to the authors, the Black-crowned Night Heron and the Cattle Egret extend to a significant number until August 2006, whereas in the present study, the number of nests registered at the beginning of August was lower. The same authors reported that the beginning of the reproductive season of Cattle Egrets was in June 2006, which reached its highest number later in August. Compared with the present study, the highest number of nests was registered in June with a decreasing trend in subsequent months. Likewise, Ibarra-Portillo et al. [Citation19] recorded in the last week of June 2006 a greater number of Snow Egret nests than those reported in the present study during the last week of June 2015. Low-abundant species like Snowy Egrets, Black-crowned Night Herons, and Great Egrets in La Barra could be being eclipsed by overabundant species such as the Neotropical Cormorant. The Neotropical Cormorant was the most abundant species in the colony and its numbers exceeded the maximum amount registered in previous years at La Barra [Citation18]. The long-term effect of the increasing high densities of co-existing heron species, the arrival of Neotropical Cormorants, and therefore the decrease in reliable nesting sites in the La Barra colony since 2001 [Citation17] may cause temporal segregation of the species to avoid interspecific competition, forcing the low-abundance ones to nest at different times than the high-abundance species, diminish in numbers, or even desert the area [Citation13,Citation37,Citation38]. Cormorant species tend to generate these dynamics. For example, Double-crested Cormorants (Phalacrocorax auritus; Lesson, 1831) in the Great Lakes region have determined the decline of coexisting ardeid species from nesting areas when the first arrived at a pre-existing heronry [Citation37]. Similarly, the effect of the post-arrival of Great Cormorants (Phalacrocorax carbo; Linnaeus, 1758) to an established breeding colony has negatively influenced the nest’s numbers of herons and a within-colony spatial and vertical segregation of the nesting species in north-western Italy [Citation39]. During the 2007 season, the numbers of Great Egrets, Snowy Egrets, and Black-crowned Night Herons in La Barra almost tripled the numbers recorded here (e.g. Great Egret = 169 and Snowy Egrets = 55) when the Neotropical Cormorant was nesting for the third time in the colony (first time of nesting was in 2005), and its numbers were relatively low [Citation18]. In this way, the arrival and, consecutively, the nesting of the Neotropical Cormorant to La Barra forest suggests a negative impact on the number and timing of the ardeid species, and even on the spatial distribution of the latter, a subject better discussed below.
The use of vegetation in the La Barra forest by the colony has changed over time. Here, more species were counted and identified than those reported in previous studies [Citation17,Citation19]. Since Ibarra-Portillo et al. [Citation19] reported only 10 trees belonging to four species in 2000, the number of trees and shrubs used for nesting has increased by 172 individuals belonging to 20 species by 2015. Moreover, the species used for nesting has changed throughout this period. For example, T. rosea was previously registered as a species used for nesting; however, a single individual approximately 2 m high was found within the colony area in this study, which could hardly host nests of any of the waterbird species due to the small size of the branches.
Albizia caribaea is another species recorded by Ibarra-Portillo et al. [Citation19], which although it exists within the La Barra forest, no trees were found within or near the nesting colony. On the contrary, S. polyphylla is a species not registered in previous studies despite being the most used species according to the present study (32 trees and shrubs) and distributed practically throughout the entire area of the nesting colony (). These divergences occur for various reasons: a lesser nest occupancy in previous years, and therefore fewer plant species used, variation in the species-based nest plant selection [Citation40], and consequents vegetation successions caused by the colony in previous years (which would therefore affect species-based nest plant selection) [Citation37,Citation41].
For the last, it is important to highlight the variation of the height per species recorded here () that supports the statement of subsequent local successions due to the colony’s presence [Citation37,Citation41], where species that previously could not develop due to sunlight restriction by the canopy are now developing, as is the case of shrub-sized and vine species registered here ().
Nesting area and densities
The total area of the colony for the 2015 reproductive season slightly differs from that recorded in La Barra by Herrera et al. [Citation18] (2.14 ha – 2.2 ha, respectively). This would indicate that the use of space in the La Barra forest has varied minimally; however, nest densities per hectare have increased here (552.72 nests/ha in 2007 vs 584.58 nests/ha in 2015). These trends have been seen in other studies, in mixed and non-mixed colonies. For example, Kazantzidis et al. [Citation16] observed an increasing nest density in an Axios Delta (Greece) heronry conformed by two species. By Bayer and McMahon [Citation42] found the same increasing density pattern (per hectare and per tree) was found in five of the seven Great Blue Heron (Ardea herodias; Linnaeus, 1758) colonies along the Oregon coast.
Densities varying through the years could be explained by the colony formation process and the structure of the habitat [Citation16,Citation43]. A mixed colony usually starts with one or a pair of species and later more are added, thus increasing the colony size to a point of stabilization and then increasing its density [Citation43]. During this process, the vegetation structure is modified with concurrent successions [Citation38], which allows different height strata and, therefore, more nesting sites availability for small species that nest at lower heights. This means that the area of the colony can vary little, but the different height strata of the vegetation allow a wide vertical distribution for nesting, thus increasing the density in relation to the area. As for the nest density per tree/shrub, this could be partially determined by the vegetation species and the number of nests [Citation9]. For example, Neotropical Cormorants were the most abundant species in the La Barra colony, and their highest densities are in trees of medium and high stratum that in general, show greater crown areas and more nesting structures availability. However, a significant but low correlation between the height of trees/shrubs and the number of nests was found during data exploration (see Results), suggesting that other variables take place when observing greater nest densities (see the “Factors influencing the within-colony distribution section” below). For example, the Cattle Egret was the second most abundant species in the La Barra colony. Despite the fact that plants used by this species were shrubs and vines (low height), the latter allows more area and concentrated structures for nesting, which is a suitable nest-site characteristic for this species that prefers to establish its nests very close to each other [Citation37].
Colony’s spatial distribution
According to the present study, there is a relationship between the nest distribution per species and the nearest neighbour species in mixed colonies. Here, and although not strictly, the Cattle Egrets, Great Egrets, and Neotropical Cormorants mainly established their nests in trees and bushes close to a conspecific neighbour, while the other species preferred to nest close to contraspecifics. However, caution may be taken here for those low-numbered species in the La Barra forest (Snowy Egret and Black-crowned Night Heron) whose contribution to the test applied here may be affected by the small number of nests counted and therefore is unreliable. Despite the above, this pattern has been seen in other waterbird colonies.
McCrimmon [Citation9] found that Great Egret nested significantly closer to other Great Egrets and for smaller species such as Cattle Egrets and Snowy Egrets, they generally nested closer to contraspecifics than to conspecifics. Naugle et al. [Citation12] found the same pattern where Great Egrets nested with conspecifics at the periphery, and Cattle Egrets and Black-crowned Night Herons tended to overlap their nesting areas. Kazantzidis et al. [Citation16] found that Black-crowned Night Herons tend to nest close to conspecifics; however, the colony was formed by two species so this suggests that patterns of nest-site selection based on the neighbour species will be determined by the nesting species that form the colony, taking into account that in mixed colonies, species have a non-random pattern and tend to nest close to conspecifics or with the species most similar in size [Citation15].
Colony’s vertical distribution
The waterbird colony studied was vertically distributed and showed a remarkable interspecific hierarchy: Great Egrets and Neotropical Cormorants, in the canopy, Neotropical Cormorants in the middle layer, Black-crowned Night Herons, Snowy Egrets, and Cattle Egrets in the lower layer. The interspecific hierarchy found here is consistent with other studies carried out in habitats with different vegetation and hydrological regimes [Citation9,Citation44–46]. The verticality of the heronries is usually determined by the species body mass, where the larger ones occupy the higher strata and the smaller ones the lower strata [Citation12].
Of the five species recorded in this study, the Great Egret is the largest, followed by the Neotropical Cormorant, Black-crowned Night Herons, Snowy Egrets, and Cattle Egrets, which suggests that species size influences the vertical distribution of La Barra nesting colony. Also, within species, temporal segregation in nesting can have a determining effect on nest location, although this variation usually occurs between conspecific or between species with similar sizes that share the same height strata. For example, Bachir et al. [Citation13] found that the first Cattle Egrets arriving at a colony selected the highest trees and the highest nest supports close to the trunk. In other cormorant species, the first nesting pairs (usually, the highest quality ones) of the season choose the best sites (central location and highest trees) due to a less predation rate and therefore greater fledgling’s survival, where the last of the season exhibit lower brood quality [Citation7,Citation10]. This site selection by the highest quality pairs of one species can even affect the selection of nesting sites of contraspecifics [Citation10]. Here, Neotropical Cormorants were nesting in different height strata (in trees >10 m) and could explain the dynamics of the La Barra colony since 2001, where in previous years, species such as Snowy Egret or Great Egret have shown a greater number of nests compared to the results of this study, so the increasing nesting of Neotropical Cormorants could be negatively affecting the other species (see ).
Factors influencing the within-colony distribution
Some of the factors influencing nest-site selection by species have been addressed and extensively discussed in the different approaches above. Previous studies suggest that higher nest densities are usually found in central sites because these better protect against predators, and therefore a preferred site for nesting [Citation6,Citation7]. However, in heterogeneous habitats, a trade-off between nesting locations from the central to periphery of the colony occurs when the first exhibits a major probability of nest collapse before the conclusion of breeding activities rather than the second, which could exhibit high-quality sites [Citation7]. Therefore, as well as in La Barra forest, waterbirds tend to occupy the best nesting structures with the highest qualities, as a proxy explained here by the higher heights of trees/shrubs. shows how the nest density distribution is not concentrated in the central zone of the colony, but quite the opposite, suggesting that nest-site selection is attributable to high-quality sites and not predator protection. Moreover, it is well known that colonies are always situated close to a reliable source of food [Citation3]. In this line, the best model for shorter distances to the Ostúa River are also determining the within-colony establishment of nests, where pairs are choosing the nesting sites closest to the food resources to minimize foraging costs. Previous studies have shown that heron colonies used to be established close to local concentrations of foraging habitat, suggesting that this dynamic is also reflected in the within-colony distribution [Citation47,Citation48].
Finally, the best model indicates that lesser species occupying the same tree/shrub are determining the high nest densities per tree/shrub. This can be partially explained by the vertical distribution and the spatial distribution results derived in this study and discussed above, where species in mixed colonies are not randomly distributed, but rather follow a spatial pattern set on by the preference to nest next to conspecifics or contraspecifics of similar size and in a determined height stratum [Citation9,Citation15,Citation46]. Thus, these findings suggest that species will prefer to nest in the same tree/bush of similarly sized individuals (conspecifics or contraspecifics) and that some dispersed pairs will nest with contraspecifics due to a different arrival timing for nesting or by the availability of suitable nesting structures [Citation7].
Management implications
Monitoring the reproductive activity of waterbirds and the vegetation associated with the La Barra nesting colony has become an important instrument for the conservation and suitable management of the area since it provides direct information regarding the habitat conditions and population dynamics of this species [Citation49–53]. This study provides several aspects of the within-colony nesting distribution but also some insights into the temporal dynamics of the breeding species and the state of the vegetation associated with the colony that is relevant for the conservation of both.
The overabundance of Neotropical Cormorants and their population trends in the La Barra colony suggests that this species is of a certain concern regarding the impact on other breeding waterbirds but also on the nesting habitat. The Neotropical Cormorant population has increased in El Salvador, and it is even considered a pest species due to human–wildlife conflicts with local artisanal fishermen on inland wetlands and the negative impact of nesting vegetation [Citation38,Citation41,Citation54–56].
This population growth can also be reflected in La Barra due to the higher reproductive success achieved in other nearby wetlands [Citation55], given by the high availability of food, association with other heronries, and its plasticity for nesting [Citation54,Citation57]. Current methods of biological control, adult and nest eradication are ongoing in the principal wetlands where this species is present [Citation58], but there is still no scientific evidence of a plausible population reduction. Future research should aim to determine temporal population trends of the Neotropical Cormorants on national scale, interspecific relations with heronries, and determine the most suitable conservation measure applications to manage this species.
As for the conservation of La Barra forest, due to its small size and different threats associated with it (see some insight of threats in Ibarra-Portillo et al. [Citation18]), the environmental institution of the government should harden the application of restrictions for wood and soil extraction to nearby villages and the waste management of pesticides packaging that is also threatening the waterbird colony. As for the illegal logging at La Barra, it is important to mention that during the species-specific bivariate correlations (see Results), some species have shown a preference to locate their nests closer to the Ostúa River in taller trees/shrubs and away from the urban area. Taller trees and shrubs would indicate more conserved areas of the forest, and therefore waterbirds are nesting as much as possible – and within the colony – away from this human perturbation.
Finally, the environmental institution must strengthen the ranger’s corps and periodicity for monitoring this forest since low-number of staff for the total surveillance of the 1881.20 ha of the National Park and the distance between the base and the study site makes it very inaccessible and vulnerable (personal observation).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Parsons KC. Heron nesting at Pea Patch Island, Upper Delaware Bay, USA: abundance and reproductive success. Colon Waterbirds. 1995;18:69–78.
- Kazantzidis S, Yfantis G, Poirazidis K. Factors influencing species composition and nest abundance of heron colonies. J Biol Res-Thessaloniki. 2013;20:276–289.
- Siegel-Causey D, Kharitonov SP. The evolution of coloniality. In: Power DM, editor. Current ornithology. New York (NY): Plenum; 1990. p. 285–330.
- Kushlan JA, Steinkamp J, Parsons KC, et al. El Plan para la Conservación de Aves Acuáticas de Norteamrica, Versión 1 [The North American Waterfowl Conservation Plan]. Washington: Waterbird Conservation for the Americas; 2002. Spanish.
- Weller M. Wetlands birds habitat resources and conservation implications. Australia: Cambridge University Press; 2003.
- Minias P. Evolution of within-colony distribution patterns of birds in response to habitat structure. Behav Ecol Sociobiol. 2014;68:851–859.
- Minias P, Kaczmarek K. Is it always beneficial to breed in the centre? Trade-offs in nest site selection within the colony of a tree-nesting waterbird. J Ornithol. 2013;154:945–953.
- Minias P, Wojczulanis-Jakubas K, Rutkowski R, et al. Spatial patterns of extra-pair paternity in a waterbird colony: separating the effects of nesting density and nest site location. Behav Ecol Sociobiol. 2016;70:369–376.
- McCrimmon DA. Nest site characteristics among five species of herons on the North Carolina coast. Auk. 1978;95(2):267–280.
- Lee HJ, Yi JH, Hung HC. Change in nest site and population size of great cormorants (Phalacrocorax carbo) in relation to different Ardeidae species in inland breeding sites in Korea. J Ecol Environ. 2019 [cited 2020 Nov 1]:7. DOI:10.1186/s41610-019-0125-4
- Blanco D. Los humedales como hábitat para aves acuáticas [Wetlands as habitat for waterbirds]. In: Malvárez AI, editor. Tópicos sobre humedales subtropicales y templados de Sudamrica [Topics on subtropical and temperate wetlands of South America]. Montevideo (Uruguay): UNESCO; 1999. p. 219–228. Spanish.
- Naugle DE, Johnson RR, Meeks WA, et al. Colonization and growth of a mixed-species heronry in South Dakota. Colon Waterbirds. 1996;19(2):199–206.
- Bachir AS, Barbraud C, Doumandji S, et al. Nest site selection and breeding success in an expanding species, the Cattle Egret Bubulcus ibis. Ardea. 2008;96(1):99–107.
- Janiszewski T, Minias P, Lesner B, et al. Age effects on reproductive success, nest-site location, and offspring condition in the Great Cormorant Phalacrocorax carbo sinensis. J Ornithol. 2017;158:193–202.
- Burger J. Resource Partitioning: nest site selection in mixed species colonies of herons, egrets and ibises. Am Midl Nat. 1979;101(1):191–210.
- Kazantzidis S, Goutner V, Pyrovetsi M, et al. Comparative Nest site selection and breeding success in 2 sympatric ardeids, Black-crowned Night-Heron (Nycticorax nycticorax) and Little Egret (Egretta garzetta) in the Axios Delta, Macedonia, Greece. Colon Waterbirds. 1997;20(3):505–517.
- Ibarra-Portillo R, Herrera N, Rivera R. Anidación de Ardea alba (Ciconiformes: ardeidae) en Lago de Guija, El Salvador y Guatemala [Nesting of Ardea alba (Ciconiformes: ardeidae) in Lago de Guija, El Salvador and Guatemala]. Mesoamericana. 2005a;9(1/2):4–7. Spanish.
- Herrera N, Pineda L, Ibarra-Portillo R, et al. Monitoreo de la avifauna del Parque Nacional San Diego La Barra [Monitoring the Birdlife of San Diego La Barra National Park]. San Salvador: Centro de Protección de Desastres (CEPRODE), Grupo de Trabajo en Conservación de Aves de El Salvador, Partners in Flight–El Salvador; 2008b. Spanish.
- Ibarra-Portillo RE, Herrera N, Aguilar B, et al. Colonia de anidación de aves, La Barra, Metapán, Santa Ana, El Salvador [Bird nesting colony, La Barra, Metapán, Santa Ana, El Salvador]. Bioma. 2014;17(2):25–36. Spanish.
- Herrera N. Evaluación ambiental del complejo Lago de Güija [Environmental Assessment of the Güija Lake complex]. San Salvador: Ministerio de Medio Ambiente y Recursos Naturales, Centro Nacional de Registro; 2005. Spanish.
- Gallo M, Ricord Z. II Informe Nacional Sistema de Áreas Naturales Protegidas, El Salvador [Second National Report System of Protected Natural Areas, El Salvador]. San Salvador: Ministerio de Medio Ambiente y Recursos Naturales; 2006. Spanish.
- Quintana P, Sermeño A III. Informe Nacional de Áreas Naturales Protegidas, El Salvador [Third National Report System of Protected Natural Areas, El Salvador]. San Salvador: Ministerio de Medio Ambiente y Recursos Naturales; 2010. Spanish.
- Stiles F, Skutch A. Guía de aves de Costa Rica [Costa Rica Bird Guide]. Heredia: Instituto Nacional de Biodiversidad; 1998. Spanish.
- Ralph CJ, Geupel GR, Pyle P, et al. Manual de métodos de campo para el monitoreo de aves terrestres [Manual of field methods for land bird monitoring]. California (CA): Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture; 1996.
- Cross T. Waterbirds. New York (NY): W.W. Norton Company; 2009.
- King KA. Colonial wading bird survey and census techniques. In: Sprunt A, Ogden JC, Winckler S, editors. Wading birds, research report 7. New York (NY): National Audubon Society; 1978. p. 155–160.
- Rodgers JA, Kubilis PS, Nesbitt SA. Accuracy of aerial surveys of waterbird colonies. Waterbirds. 2005;28(2):230–237.
- Garrigues R, Dean R. The birds of Costa Rica A field Guide. Ithaca: Zona Tropical; 2014.
- Larjavaara M, Muller-Landau HC. Measuring tree height: a quantitative comparison of two common field methods in a moist tropical forest. Meth Ecol Evolut. 2013;4(9):793–801.
- UICN. Mapping standards and data quality for the IUCN Red List categories and criteria. 2018. Online. Available from: https://nc.iucnredlist.org/redlist/resources/files/1539098236-Mapping_Standards_Version_1.16_2018.pdf
- R Core Team. R: a language and environment for statistical computing. Version 3.6.3. Vienna: R Foundation for Statistical Computing; 2020. Online. Available from: https://www.R-project.org/
- Silverman BW. Density Estimation. London: Chapman and Hall; 1986.
- Agresti A. Inference for contingency tables. In: Agresti A, editor. Categorical data analysis. 2nd ed. New Jersey (NJ): A John Wiley and Sons, Inc. Publication; 2002. p. 70–114.
- Meyer D, Zeileis A, Hornik K, et al. vcd:visualizing categorical data. v. 1.4-8; 2015. Available from: https://cran.r-project.org/web/packages/vcd/index.html
- Zuur AF, Ieno EN, Walker NJ, et al. Mixed effects models and extensions in ecology with R: statistics for biology and health. New York: Springer; 2009b.
- Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol. 2009a;1:3–14.
- Arendt WJ, Arendt AI. Aspects of the breeding biology of the Cattle Egret (Bubulcus ibis) in Montserrat, West Indies, and its impact on nest vegetation. Colon Waterbirds. 1988;11(1):72–84.
- Cuthbert FJ, Wires LR, McKearnan JE. Potential impacts of nesting Double-crested Cormorants on Great Blue Herons and Black-crowned Night-Herons in the U.S. Great Lakes region. J Great Lakes Res. 2002;28(2):145–2154.
- Gagliardi A, Casola D, Preantoni D, et al. Unwanted guest in heronries: the influences of Great Cormorant Phalacrocorax carbo on nest site selection of Grey Heron Ardea cinerea in northwestern Italy. Cormorant Res Group Bullet. 2015;8:36–38.
- Custer TW, Osborn RG, Stout WF. Distribution, species abundance, and nesting-site use of Atlantic coast colonies of herons and their allies. Auk. 1980;97:591–600.
- Craig EC, Elbin SB, Danoff-Burg JA, et al. Impacts of Double-Crested Cormorants (Phalacrocorax auritus) and other colonial waterbirds on plant and arthropod communities on Islands in an urban estuary. Waterbirds. 2012;35(suppl.1):4–12.
- Bayer DR, McMahon E. Colony sizes and hatching synchrony of Great Blue Herons in coastal Oregon. Murrellet. 1981;62:73–79.
- Kharitonov SP, Siegel-Causey D. Colony formation in seabirds. In: Johnston RF, editor. Current ornithology. New York (NY): Plenum; 1988. p. 223–272.
- Cupul-Magaña FG. Observaciones sobre la anidación de tres especies de ardéidos en el estero Boca Negra, Jalisco, México [Observations on the nesting of three species of ardeidae in the Boca Negra estuary, Jalisco, Mexico]. HUITZIL. 2004;5(1):7–11. Spanish.
- Rodríguez-Barrios J, Troncoso F. Éxito de anidación de la garza real Egretta alba (Aves, Ardeidae) en el departamento de Córdoba, Colombia [Nesting success of the Gray Heron Egretta alba (Aves, Ardeidae) in the department of Córdoba, Colombia]. Acta Biol Colomb. 2006;11(1):111–121. Spanish.
- Ayaş Z. Nest site characteristics and nest densities of Ardeids (Night Heron: nycticorax nycticorax, Grey Heron: Ardea cinerea, and Little Egret: Egretta garzetta) in the Nallıhan Bird Sanctuary (Sarıyar Reservoir, Ankara, Turkey). Turk J Zool. 2008;32:167–174.
- Gibbs JP. Spatial relationships between nesting colonies and foraging areas of Great Blue Herons. Auk. 1991;108(4):764–770.
- Gibbs JP, Kinkel LK. Determinants of the size and location of Great Blue Heron colonies. Colon Waterbirds. 1997;20(1):1–7.
- Custer TW, Osborn RG. Wading birds as biological indicators: 1975 colony survey. Washington: U.S. Dept. of Interior, Fish and Wildlife Service; 1977.
- Kushlan JA. Colonial waterbirds as bioindicators of environment change. Colon Waterbirds. 1993;16(2):223–251.
- Kushlan JA. The conservation of wading birds. Colon Waterbirds. 1997;20(1):129–137.
- Martin TE, Geupel GR. Nest-monitoring plots: methods for locating nests and monitoring success. J Field Ornithol. 1993;64(4):507–519.
- Villareal J, Jiménez A. Colonia de garzones (Mycteria americana) en un paisaje agrosilvopastoril del bosque seco, Costa Rica: implicaciones en conservación [Colony of Great Egrets (Mycteria americana) in an agrosilvopastoral landscape of the dry forest, Costa Rica: implications for conservation]. Zeledonia. 2008;12(2):8–16. Spanish.
- Herrera N, Ibarra Portillo RE, Salinas M. Distribución, abundancia y anidación del cormorán neotropical (Phalacrocorax brasilianus) en El Salvador [Distribution, abundance and nesting of the neotropical cormorant (Phalacrocorax brasilianus) in El Salvador]. Mesoamericana. 2008a;12(1):24–31. Spanish.
- Herrera N, Hernández J, Vega I, et al. Población anidante e impacto en la pesca artesanal del cormorán neotropical Phalacrocorax brasilianus (Suliformes: phalacrocoracidae), en el sitio Ramsar Cerrón Grande, El Salvador [Nesting population and impact on artisanal fishing of the Neotropical cormorant Phalacrocorax brasilianus (Suliformes: phalacrocoracidae), at the Cerrón Grande Ramsar site, El Salvador]. Revista Comunicaciones Científicas y Tecnológicas. 2015;1(1):9–18. Spanish.
- Ayers CR, Hanson‐Dorr KC, O’Dell S, et al. Impacts of colonial waterbirds on vegetation and potential restoration of Island habitats. Restor Ecol. 2005;23(3):252–260.
- Hanson KC, De Vault TL, Dinsmore SJ. Increased abundance and first breeding record of the Neotropic Cormorant (Phalacrocorax brasilianus) on the Alluvial Plain of Mississippi. Southeast Nat. 2010;9(2):385–394.
- Pineda LA. Guía para manejar y controlar el Pato Chancho (Phalacrocorax brasilianus) en los humedales de El Salvador [Guide to manage and control the Neotropical cormorant (Phalacrocorax brasilianus) in the wetlands of El Salvador.]. San Salvador: Ministerio de Medio Ambiente y Recursos Naturales; 2018. Spanish.