ABSTRACT
Freshwater macroinvertebrates play an important role in maintaining stream food webs. Odonata (dragonflies and damselflies) are important top predators in these communities and serve as indicators of stream health. Our understanding of odonate assemblages is limited in the Caribbean and the natural history of most odonate species in the region remains unknown. The focus of this research is to study the natural history of odonate species in headwater montane streams following major hurricane impacts in Puerto Rico. We monitored assemblages from August 2018 to July 2019 in two headwater streams within El Yunque National Forest, Puerto Rico. The study streams drain a protected forest, with aseasonal precipitation patterns, relatively constant water temperature, and flashy hydrographs that quickly respond to rain events. We sampled 226 adults and 550 larvae, dominated by three Caribbean endemics: Scapanea frontalis, Macrothemis celeno, and Telebasis vulnerata. Only S. frontalis and M. celeno were abundant enough to assess the temporal patterns and their natural history. Larval density fluctuated throughout the year with short peaks in abundance during different times of the year, according to the species. Small individuals (≤10 mm body length) were more abundant than the large ones. However, all size classes were present during the year. The dominant species, S. frontalis and M. celeno, had continuous development patterns, without identifiable size classes and multiple overlapping generations. The exception was the last stadium that formed a separate group in the body length vs head width plots. Species had clear habitat preferences; S. frontalis was abundant in riffles and preferred areas with high amounts of cobble. Macrothemis celeno prefers pool habitats with fine substrates. While we found trends for negative relations between abundance and discharge, canopy cover, water temperature, and rainfall, none was statistically significant. Observed patterns suggest a lack of strong temporal seasonality in the natural history of Odonata, which coincides with the aseasonal environment of streams draining our study area. Overall, our study is the first to assess temporal variability of Odonata assemblages in montane streams of Puerto Rico and provides information on Caribbean endemic species.
Introduction
Aquatic macroinvertebrates are important components of stream ecosystems and play key roles in the functioning of stream food webs [Citation1]. Like other organisms, macroinvertebrate assemblage composition is determined by abiotic and biotic factors [Citation2,Citation3]. Abiotic factors, such as temperature, water flow, and substrate composition, create an environmental scenario that ultimately determines the occurrence of a species in a habitat [Citation4]. It has been demonstrated that an increase in precipitation results in flood disturbances that couldin turn decrease the abundance of many aquatic insects [Citation5,Citation6]. Similarly, biotic factors, such as predator–prey interactions and resource competition, may also shape the distribution of a species [Citation4]. For example, macroinvertebrate abundance is often affected by fish predation [Citation7]. Studies using fish exclusion have found that, in their absence, predators, such as Odonata, can further impact invertebrate assemblages [Citation8–10].
Temporal variation of environmental variables is another factor that influences the dynamics of organisms. In the tropics, seasonality in precipitation is a well-known temporal component that results in patterns of wet and dry seasons. Macroinvertebrates inhabiting streams are then subjected to either increases in precipitation that result in floods or reductions in precipitation that cause low-flow conditions. Consequently, some groups have a peak of emergence during the dry season, while others have peaks during the wet season [Citation11]. In seasonal tropical regions, the abundance of some groups of aquatic macroinvertebrates increases during the dry season, while it decreases during the wet season [Citation5]. Decreases in abundance could be attributed to the high mortality of organisms after flooding events [Citation12]. Thus, seasonality of environmental factors is important in determining species richness and abundance in tropical communities.
Odonates are useful model organisms to study the effects of abiotic factors on macroinvertebrate assemblage composition and abundance as they are present in both terrestrial and aquatic habitats. In temperate regions, odonates are mostly controlled by seasonality in temperature. For example, Wissinger [Citation8] studied larval odonate assemblage dynamics only during the nine ice-free months of the year where odonates were available. In the seasonal tropics, Odonata are subjected to the potential loss of waterbodies brought on by the dry season. Some species, such as Cora marina, adapt to these conditions by ovipositing in wet logs to prevent egg desiccation and ensure the survival of small larvae [Citation13]. Other factors influencing odonate assemblages are changes in stream size. It has been found that sister species use habitat partitioning and distribute themselves along the lower, middle, and upper reaches of a stream gradient depending on their habitat preference [Citation14].
Hurricanes, although infrequent, are major disturbances that alter the environment, creating new environmental conditions that affect biotic communities. Important components of ecosystems, such as canopy cover, nutrient cycling, and litter fall, are modified and have implications on the established biota [Citation15]. In some forest ecosystems, modifications of habitat structure brought on by hurricanes cause the displacement of native species and the establishment of invasive ones [Citation15,Citation16]. In streams, increases in deposited organic matter can have positive effects for the recruitment of consumers, such as shrimps [Citation17]. For odonates, forests are prime locations because they provide shaded areas, high humidity, protection from environmental factors (e.g. extreme temperatures), mating opportunities and oviposition sites [Citation18]. However, studies on the effect of hurricanes on odonate assemblages are limited. In the United States, after Hurricane Katrina made landfall, it was found that even though abandoned pools were colonized by mosquito larvae and other organisms, their Odonata predators did not influence mosquito presence [Citation19].
Here, we study the relationship between environmental factors and odonate assemblages in aseasonal rain forest streams following major habitat alterations created by the impact of Hurricanes Irma and María in Puerto Rico. The objectives are to (1) evaluate temporal patterns in Odonata abundance and composition and (2) assess the influence of environmental factors on those patterns including canopy cover heterogeneity over the streams caused by the hurricanes. El Yunque National Forest is one of the most preserved and ecologically studied areas in the Caribbean but lacks information on odonate assemblages. Based on precipitation and discharge patterns, we expect that larval abundance will be closely related to stream discharge. We also expect that areas with open canopies will have high abundances of adult odonates, as most species require sunlight for thermoregulation.
Materials and methods
Study site
The study was conducted in El Yunque National Forest, in northeastern Puerto Rico (). Specifically, we selected two streams within the Luquillo Long-Term Ecological Research (LTER) site. The region is classified as a tropical wet forest, receives an average of 3,500 mm of precipitation every year and has an annual air temperature of approximately 25°C [Citation20]. It exhibits little seasonality, with drier periods from January to April, but it is considered an aseasonal forest [Citation21,Citation22]. The study streams drain Tabonuco forest (Dacryodes excelsa Vahl.), with palms (Prestoea acuminata var. montana (Graham)) dominating riparian vegetation. Substrates are predominantly large boulders and cobbles, except for pool areas where finer substrates, like sand and silt, can be found [Citation23].
Figure 1. Map of study site. a) The archipelago of Puerto Rico, b) El Yunque National Forest (dark grey) located on the northeastern part of the Island and c) El Verde Field Station (triangle), the closest location to all study streams.
At the time, this study was conducted, Puerto Rico was still recovering from the impacts of two major hurricanes. The first, Hurricane Irma, remained north as it passed close to the Island on 6 September 2017. Hurricane María struck land on 20 September 2017 near the municipality of Yabucoa at 10:15 UTC −4 as a category 4 hurricane and caused catastrophic damage throughout the Island. Sustained winds of 103 km/h were reported in San Juan, before the hurricane made landfall on the Island [Citation24]. Climatic conditions in streams at El Yunque National Forest were completely altered after the hurricanes, with abrupt changes in canopy and vegetation that resulted in more solar radiation, and higher temperatures.
Odonata sampling
We assessed temporal patterns of species of Odonata in two second-order streams, Quebrada Prieta and Quebrada Gatos, with elevations of 350 m and 206 m, respectively. Temporal changes were studied monthly from August 2018 to July 2019. All adult specimen surveys were conducted between 09:00 and 15:00 h during sunny days to ensure activity. Adults were surveyed for 45 minutes, always by the same observer, along 100 m transects, and their abundance was recorded. A 1.5 m entomological net was used to flush adults resting in nearby vegetation. Other studies have successfully used 100 m transects since it manages to represent the Odonata community well [Citation25,Citation26]. Furthermore, 45 minutes is more than enough time considering the Island’s diversity and the low adult abundances previously recorded in our study streams [Citation27]. Species that could not be visually identified were captured with entomological nets and identified at the laboratory using available taxonomic keys and species descriptions [Citation28,Citation29]. We used the same 100 m transects for larval collection. We randomly selected five 5 m segments to be sampled for 15 minutes using aquarium fish nets. In each segment, we sampled the same number of pools and riffles if they were available. Larvae were sorted from benthic material and preserved in 70% ethanol for later identification. At each sampling, similar to Peck et al. [Citation30], we measured environmental variables, such as canopy cover (using a spherical densiometer), water and air temperature, and characterized the substrate of each segment (percent of boulder, cobble, gravel, sand and silt). Streamflow and precipitation data were obtained from LTER monitoring stations. We used El Verde precipitation data; the monitoring point is ~500 m from Prieta and ~1000 m from Gatos (https://luq.lter.network/data/luqmetadata14). Stream discharge is monitored continuously in Prieta only, and we applied these data to both streams, assuming that Gatos behaves similar to Prieta with respect to changes in discharge (https://luq.lter.network/data/luqmetadata182). Water chemistry variables such as pH, conductivity, and turbidity were not measured since our study streams are in a protected forest and these variables are relatively stable [Citation31].
Larval head width and body length were measured using a dissecting microscope (0.7X or 1.0X) equipped with an AmScope digital camera and software (AmScope 3.7). Specimens were arranged in petri dishes and photographed for measurements. Larger specimens, especially those with developed and visible wing sheaths, were identified using the key in Neiss et al. [Citation32]. Smaller larvae could not be identified with taxonomic keys. To classify them, specimens were arranged in size sequences according to their characteristics, such as eye shape and body form, and assigned to one of the known species for the study site.
Data analysis
Adult and larval abundances and environmental variables were tested for normality and transformed if necessary to determine if a parametric or non-parametric test should be used. We performed t-tests to determine if there were significant differences between species richness and abundance among habitats and streams. Additionally, we performed a Kruskal–Wallis test to identify if there were significant differences in adult abundances in each study stream. Linear regressions were applied to relate the main environmental variables with odonate abundance. An analysis of variance was performed to detect differences between adult species abundances. All analyses were run using PAST version 4.02 [Citation33].
Results
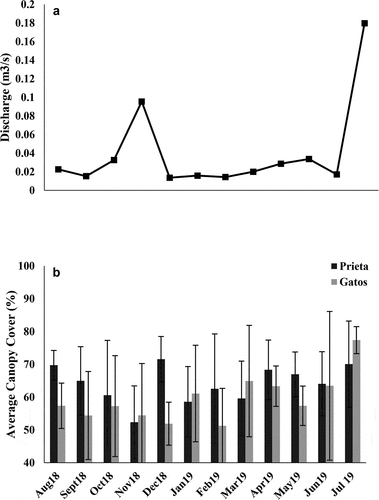
Quebrada Prieta and Quebrada Gatos had similar air (t = 0.36 p = 0.715) and water (t = 0.84, p = 0.409) temperature patterns (). August had the highest air temperatures with 28.8°C and 28.4°C for Prieta and Gatos, respectively. The lowest temperatures were recorded in January with 21.8°C and 21.3°C for Prieta and Gatos, respectively (). The highest water temperatures were recorded in July, with temperatures of 24.2°C and 24.1°C for Prieta and Gatos, respectively. The lowest temperatures were recorded in December and January, with temperatures ranging in the 20.0°C (). Stream discharge ranged from 0.017 to 0.059 m3/s in a year. Peaks in discharge were observed in November 2018 and July 2019 with values 0.058 m3/s and 0.059 m3/s, respectively. The periods with low discharge values were observed from December 2018 to July 2019 (). Canopy cover over the stream channel was highly variable and ranged from 50% to 70% (). We observed new growing riparian vegetation (e.g. ferns, Cecropia trees) that although they were not as high as tree canopies, were high enough to create shade conditions over the streams.
Figure 2. Average monthly air (a) and water (b) temperatures from August 2018 to July 2019. Error bars represent the standard deviation.
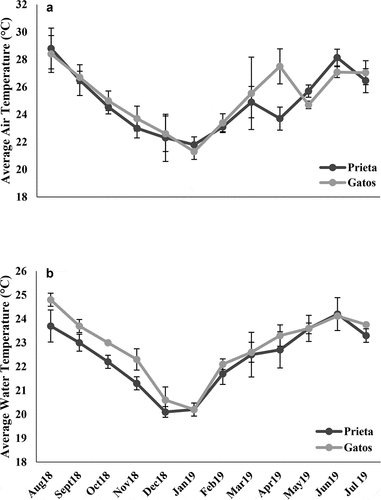
Figure 3. Monthly average stream discharge (m3/s) in Quebrada Prieta (A) and average percent of monthly canopy cover over the streams from August 2018 to July 2019 (B). Error bars represent the standard deviation.
We observed 226 adults during the year of sampling. The species recorded were as follows: Scapanea frontalis (Burmeister, 1839), Macrothemis celeno (Selys in Sagra, 1857), Dythemis rufinervis (Burmeister, 1839), Telebasis vulnerata (Hagen 1861), Enallagma coecum (Hagen, 1861), and an unidentified species of Triacanthagyna that evaded capture (). In both streams, T. vulnerata was more frequently encountered than any of the other species. Abundances of T. vulnerata were significantly higher in Quebrada Prieta than in Quebrada Gatos (t = 5.53, p < 0.001; ). The adult abundances of the different species were significantly different only in Quebrada Prieta (Kruskal-Wallis, p < 0.001), while for Quebrada Gatos, it was not significant (Kruskal-Wallis, p = 0.255).
Figure 4. Adult abundance (a) and larvae abundance for all species collected from August 2018 to July 2019 in Quebrada Prieta and Quebrada Gatos. Peaks in larval abundance were observed in October 2018 and June 2019 (b).
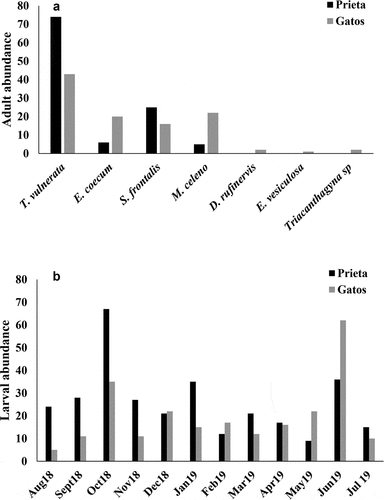
We found that larval abundance was higher in relation to their adult counterparts. We collected a total of 550 larvae during the year of sampling. In both streams, larval assemblages were mainly composed of two species of suborder Anisoptera: S. frontalis and M. celeno. It is worth mentioning that S. frontalis and M. celeno are the only species in their respective genera found in Puerto Rico, which facilitated identification. We collected 412 specimens of S. frontalis and 101 specimens of M. celeno. Both species were consistently found during our monthly sampling. Additionally, we found 37 Coenagrionidae specimens, but these were only collected in October 2018 and June 2019 and were too small to identify to lower taxonomic categories.
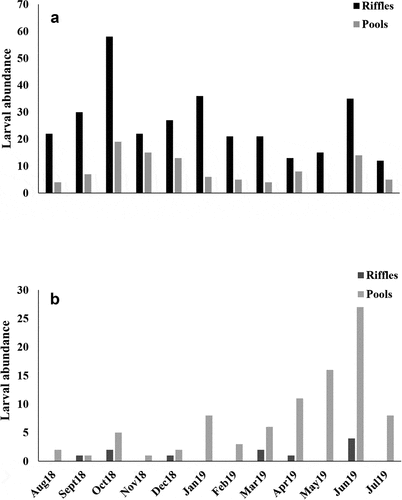
Quebrada Prieta and Gatos had similar larval abundances (t = 1.40, p = 0.174; ). We also observed that larval assemblages were more abundant in riffles than in pool meso habitats in Prieta (t = 2.15, p = 0.042; ). No significant difference was found between the habitats in Gatos (). The larval abundance of S. frontalis was higher in Prieta than Gatos (t = 3.16, p = 0.004; ). The opposite was found for M. celeno larvae, which were more abundant in Gatos than Prieta (z = 3.56, p < 0.001; ). In addition, S. frontalis preferred riffle habitats with fast water currents (t = 4.38, p < 0.001; ), while M. celeno preferred pool habitats with riparian vegetation (z = 3.37, p < 0.001; ).
Figure 5. Total larval abundance for all the species collected in Quebrada Prieta (a) and Quebrada Gatos (b) from August 2018 to July 2019.
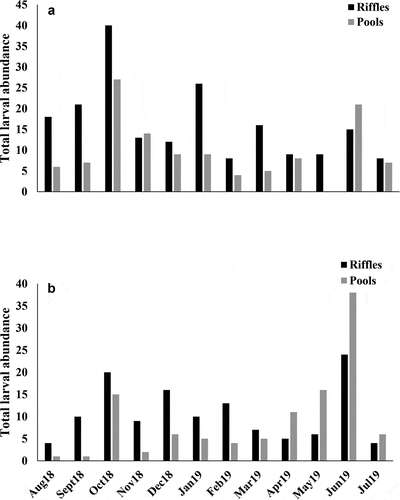
Figure 6. Abundance of Scapanea frontalis (a) and Macrothemis celeno (b) larval specimens collected in Prieta and Gatos streams.
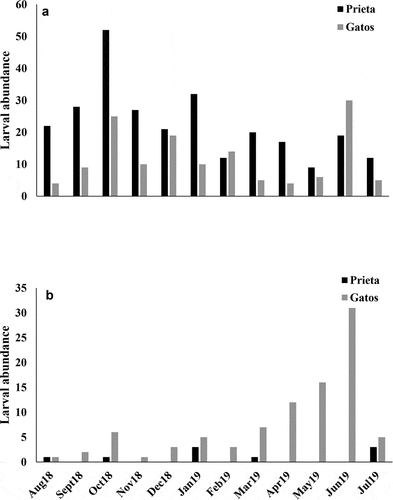
Figure 7. Abundance of Scapanea frontalis (a) and Macrothemis celeno (b) in pools and riffles in Quebrada Prieta and Quebrada Gatos. October 2018 and June 2019 had peaks in abundances for S. frontalis and M. celeno, respectively.
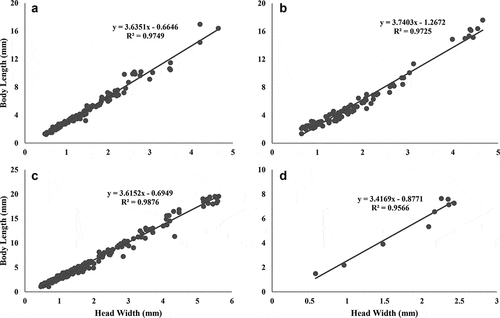
The larval density fluctuated throughout the year with a peak in abundance of specimens of S. frontalis in October 2018 in Quebrada Prieta () and a peak of M. celeno in June 2019 in Quebrada Gatos (). In both streams, small individuals (≤10 mm body length) were always more abundant than large ones (>15 mm body length), but all size classes were present during the year (). Abundances of large S. frontalis increased from January to June 2019, while large M. celeno were found from March to June 2019 (). Scapanea frontalis and M. celeno had similar development presenting a continuous increase in body size with no grouping identifiable among early growth stages. The exception was the last stadium that formed a separate group in the body length vs head width plots (). The smallest S. frontalis larvae collected measured 1.07 mm in body length, while the largest measured 19.55 mm. For M. celeno, the smallest larvae collected measured 1.35 mm in body length, while the largest measured 17.60 mm.
Figure 8. Scapanea frontalis (a) and Macrothemis celeno (b) size distribution from August 2018 to July 2019. Both species had individuals present all year with higher abundances of larger individuals at the beginning of the year. Smaller individuals (≤ 2 mm head width) were the most abundant.
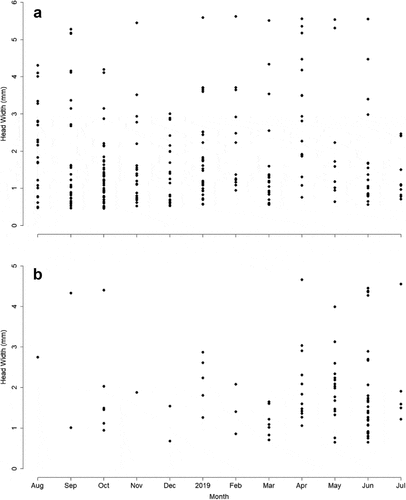
Figure 9. Relationship of head width and body length of specimens found in riffles and pools. Scapanea frontalis larvae in Gatos (a), Macrothemis celeno larvae in Gatos (b), S. frontalis in Prieta (c) and M. celeno in Prieta (d).
We found a positive relation between S. frontalis abundance in riffles and average percent of cobble substrate (R2 = 0.45, p = 0.015). This coincides with our observations as S. frontalis preferred habitats with fast flowing waters and larger substrates. There were also negative trends between larval abundances and discharge, canopy cover, water temperature, and rainfall, but none were statistically significant ().
Table 1. Summary statistics for regressions between Scapanea frontalis and Macrothemis celeno larval abundance and environmental variables
Discussion
Studies related to the ecology and life history patterns of Odonata in Puerto Rico are limited. The last publication addressing the life history of a species on the island was published in the 1940s by Needham [Citation34]. Our research is the first study assessing the temporal dynamics of Odonata populations in Puerto Rico. We found that these forest streams are dominated by three species: S. frontalis, M. celeno (Anisoptera) and T. vulnerata (Zygoptera). These species are endemic to the Caribbean and are continuously present during the year, with multiple overlapping generations, indicating a multivoltine life history. Our findings indicate that habitat characteristics are the main factors affecting the dynamics of larval Odonata in these streams.
Puerto Rico has no reported endemic species, but the dominant species found in our study are all endemic to the Greater Antilles [Citation35]. Scapanea frontalis is the only species in the genus, while M. celeno belongs to a genus with over 40 species and with a wide distribution, ranging from the southern part of the United States to Argentina [Citation28]. Telebasis vulnerata belongs to a genus that has over 50 species, with a broad distribution from the United States to Argentina [Citation29]. All three species are characteristic of forested areas, preferring streams with dense riparian vegetation. Our study highlights the importance of forest conservation to preserve these and other species distinctive of the area. This study is the first to highlight the life history patterns of these species and their development.
There are 48 species of Odonata reported for Puerto Rico [Citation27]. Of these, we recorded six species in a year of sampling: S. frontalis, M. celeno, D. rufinervis, T. vulnerata, E. coecum, and Triacanthagyna sp. Some of the species are considered habitat generalists, such as E. coecum, and have been reported in Puerto Rico at different elevations and in streams with various levels of impact [Citation35–37]. Other species are specialists of montane streams, such as S. frontalis, which is mostly found at high elevations in forested areas in Puerto Rico [Citation37]. The diversity of Odonata in our study could be considered low relative to that reported for low elevation areas, as patterns of precipitation and temperature in the mountains can restrict the establishment of species that prefer open areas with high sunlight [Citation38,Citation39]. In Puerto Rico, studies in the lowlands, even in urbanized areas, have reported higher species diversity than our study, potentially due to high habitat heterogeneity and the presence of species that are habitat generalists [Citation40].
Over time, the same dominant species were consistently observed during the year: S. frontalis, M. celeno, and T. vulnerata. Assessment of larval and adult presence indicates a lack of seasonal patterns for all species, which is potentially the result of the aseasonal nature of this region. The larval abundances of S. frontalis and M. celeno provided contrasting patterns, with brief peaks of abundance at different times during the year. However, those peaks were short-lived and might reflect natural variability. Insect studies in other aseasonal regions have reported similar findings. Studies with mayflies in forested streams in Costa Rica have reported temporal variability in abundance that does not follow a seasonal pattern [Citation41]. In contrast, tropical studies conducted in areas with clear dry-wet seasonality find more defined patterns in abundance. For example, in a dry forest of Costa Rica, Pritchard [Citation13] found marked differences in larval abundance over time and a univoltine life cycle. Similarly, Alves-Martins et al. [Citation42] report evidence of seasonality in activity and abundance of tropical savanna ponds in Brazil.
Habitat preferences differed for the larvae of the dominant species. Scapanea frontalis inhabits areas of flowing water, with a preference for cobble substrate, and M. celeno prefers slow-moving waters surrounded by vegetation. Similar observations were reported by García-Díaz [Citation37] in other montane streams in Puerto Rico. Although Quebrada Prieta and Quebrada Gatos are similar in their size and location, they had different availability of pools and riffles. Prieta had faster water currents with many riffles available, while in Gatos water currents were slower with more pools available. These differences might be an explanation as to why S. frontalis was more abundant in Prieta, while M. celeno was more abundant in Gatos. The presence of Coenagrionidae larvae was recorded within 2 months of sampling and although specimens were too small to be identified, they were likely T. vulnerata as this was the most abundant of the adult species. On both occasions, they were collected from pool habitats in shallow waters surrounded by dense vegetation. The presence of vegetation aids larvae with camouflage and protection from predators, which T. vulnerata needs as it is sharing its habitat with two dominant species [Citation43–46].
The dominant species in our study streams have asynchronous larval development and overlapping generations present at the same time. This pattern is to be expected for Odonata in tropical regions with warmer temperatures and a lack of seasonality [Citation47]. Using head widths and body lengths, we were able to identify a continuous growth pattern in the larvae of both dominant species, except for the last stadium, which was clearly identifiable. Similar reports are available for other species inhabiting waterbodies with constant warm water temperatures. For example, Argia vivida inhabiting warm Sulphur pools in Canada have continuous development without clear identifiable stages, except for the last stadium [Citation48]. Therefore, our findings for continuous development are potentially the result of the constant water temperature in these tropical headwater streams. The last stadium experiences a series of transformations as the larvae develop new structures necessary for the transformation to the adult stage and terrestrial life, thus explaining the difference in the relationship with previous larval stadiums.
Of all the environmental variables measured, we only identified a significant relationship between cobble substrate and S. frontalis larvae. As this is an aseasonal forest [Citation21,Citation22], we find little variability in environmental variables, which might explain why we found no other relationships. The lack of relationship between discharge and larval abundance suggests that our study species are adapted to abrupt changes in flow. For many species in the neotropics, their life cycles are regulated by variations in precipitation [Citation13,Citation49,Citation50]. S. frontalis and M. celeno appear to have adapted to these changes, and we would expect other species in the forest to have similar development as they are subjected to the same conditions. A continuous larval development makes sense for a place where precipitation is a constant abiotic pressure.
Overall, our study is the first to assess temporal variability of Odonata assemblages in montane streams of Puerto Rico. We observed clear patterns of habitat preference by the dominant species and limited fluctuations in their abundance throughout the year. We provide information on the life history of three endemic species in the Caribbean. This study is an important step in the study of Odonata in Puerto Rico and will support future investigations focusing on other species and their development.
Author contributions
All authors participated in the study. The study is the master’s research work of AMR, and she led all stages of the process. NMB played a key role in data acquisition, and both NMB and AR participated in data analysis and interpretation. All authors contributed to the intellectual content of the manuscript.
Acknowledgments
Our study was made possible thanks to the logistical support of El Verde Field Station and the Luquillo Long-Term Ecological Research program. We appreciate the field help by Jesus Gómez and Eric Torres. Special thanks to Javier Muzón for reviewing the taxonomic identification of larval specimens. This study was partially funded by the Luquillo LTER program (US National Science Foundation DEB-1546686).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Vannote RL, Minshall GW, Cummins KW, et al. The river continuum concept. Can J Fisheries Aquat Sci. 1980;37(1):130–137.
- van Hattum B, Timmermans KR, Govers HA. Abiotic and biotic factors influencing in situ trace metal levels in macroinvertebrates in freshwater ecosystems. Environ Toxicol Chem. 1991;10(2):275–292.
- Hussain QA, Pandit AK. Macroinvertebrates in streams: a review of some ecological factors. Int J Fish Aquaculture. 2012;4(1):114–123.
- Power ME, Stout RJ, Cushing CE, et al. Biotic and abiotic controls in river and stream communities. J North Am Benthological Soc. 1988;7(4):456–479.
- Flecker AS, Feifarek B. Disturbance and the temporal variability of invertebrate assemblages in two Andean streams. Freshwater Biol. 1994;31(2):131–142.
- Bispo PDC, Oliveira LG, Bini LM, et al. Ephemeroptera, Plecoptera and Trichoptera assemblages from riffles in mountain streams of Central Brazil: environmental factors influencing the distribution and abundance of immatures. Braz J Biol. 2006;66(2B):611–622.
- Dixon SM, Baker RL. Effects of size on predation risk, behavioural response to fish, and cost of reduced feeding in larval Ischnura verticalis (Coenagrionidae: odonata). Oecologia. 1988;76(2):200–205.
- Wissinger SA. Life history and size structure of larval dragonfly populations. J North Am Benthological Soc. 1988;7(1):13–28.
- Batzer DP, Pusateri CR, Vetter R. Impacts of fish predation on marsh invertebrates: direct and indirect effects. Wetlands. 2000;20(2):307–312.
- Hopper KR. Flexible antipredator behavior in a dragonfly species that coexists with different predator types. Oikos. 2001;93(3):470–476.
- Tumwesigye C, Yusuf SK, Makanga B. Structure and composition of benthic macroinvertebrates of a tropical forest stream, River Nyamweru, western Uganda. Afr J Ecol. 2000;38(1):72–77.
- Keke UN, Arimoro FO, Auta YI, et al. Temporal and spatial variability in macroinvertebrate community structure in relation to environmental variables in Gbako River, Niger State, Nigeria. Trop Ecol. 2017;58(2):229–240.
- Pritchard G. The life history of a tropical dragonfly: cora marina (Odonata: polythoridae) in Guanacaste, Costa Rica. J Tropical Ecol. 1996;12(4):573–581.
- Dijkstra KDB, Lempert J. Odonate assemblages of running waters in the Upper Guinean forest. Archiv für Hydrobiologie. 2003;157(3):397–412.
- Waide RB. Summary of the response of animal populations to hurricanes in the Caribbean. Biotropica. 1991;23(4):508–512.
- Lugo AE. Visible and invisible effects of hurricanes on forest ecosystems: an international review. Austral Ecol. 2008;33(4):368–398.
- Covich AP, Crowl TA, Johnson SL, et al. Post-Hurricane Hugo increases in atyid shrimp abundances in a Puerto Rican montane stream. Biotropica. 1991;23(4):448–454.
- Paulson D The importance of forests to neotropical dragonflies . Forests and Dragonflies (Pensoft Publishers). , Rivera Cordero, A, 79–101. 2005.
- Caillouët KA, Carlson JC, Wesson D, et al. Colonization of abandoned swimming pools by larval mosquitoes and their predators following Hurricane Katrina. J Vector Ecol. 2008;33(1):166–173.
- Brown S, Lugo AE, Silander S, et al. (1983). Research history and opportunities in the Luquillo Experimental Forest. Gen. Tech. Rep. SO-44. New Orleans, LA: US Dept of Agriculture, Forest Service, Southern Forest Experiment Station. 132 44.
- Zimmerman JK, Covich AP. Damage and recovery of riparian Sierra palms after Hurricane Georges: influence of topography and biotic characteristics. Biotropica. 2007;39(1):43–49.
- Gutiérrez-Fonseca PE, Ramírez A, Pringle CM, et al. When the rainforest dries: drought effects on a montane tropical stream ecosystem in Puerto Rico. Freshwater Sci. 2020;39(2):000.
- Masteller EC, Buzby KM. Composition and temporal abundance of aquatic insect emergence from a tropical rainforest stream, Quebrada Prieta, at El Verde, Puerto Rico. Introduction J Kansas Entomol Soc. 1993;66(2):133–139.
- Pasch RJ, Penny AB, Berg R. National Hurricane center tropical cyclone report: hurricane Maria. TROPICAL CYCLONE REPORT AL152017, National Oceanic and Atmospheric Administration and the National Weather Service, 1–48. 2018.
- Monteiro-Júnior CDS, Juen L, Hamada N. Effects of urbanization on stream habitats and associated adult dragonfly and damselfly communities in central Brazilian Amazonia. Landscape Urban Plann. 2014;127:28–40.
- Batista JD, Ferreira VRS, Cabette HSR, et al. Sampling efficiency of a protocol to measure Odonata diversity in tropical streams. PloS one. 2021;16(3):e0248216.
- Ramírez A, Maldonado-Benítez N, Mariani-Ríos A, et al. Dragonflies and damselflies (Odonata) from Puerto Rico: a checklist with notes on distribution and habitat. PeerJ. 2020;8:e9711.
- Garrison RW, von Ellenrieder N, and Louton JA. Dragonfly genera of the New World: an illustrated and annotated key to the Anisoptera. Baltimore: JHU Press; 2006.
- Garrison RW, von Ellenrieder N, and Louton JA. Damselfly Genera of the New World: an illustrated and annotated key to the Zygoptera. Baltimore: JHU Press; 2010.
- Peck DV, Lazorchak JM, and Klemm DJ (U.S. Environmental Protection Agency) Western pilot study field operations manual for wadeable streams. 2001.
- McDowell W (2021) Chemistry of stream water from the Luquillo Mountains ver 4923056. Data Initiative. https://doi.org/10.6073/pasta/0a09f5aa2e6f11451553c92b102279a6
- Neiss UG, Fleck G, Pessacq P, and Tennessen, K. J. Odonata: superfamily Libelluloidea Hamada, Neusa, Thorp, James H., and Rogers, D. Christopher. In: Thorp and Covich’s Freshwater Invertebrates. Academic Press; 2018. p. 399–447.
- Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4(1):9.
- Needham JG. Life history notes on some West Indian Coenagrionine dragonflies (Odonata). J Agric Univ Puerto Rico. 1941;25(3):1–18.
- Paulson DR. Critical species of Odonata in the Neotropics. Int J Odonatol. 2004;7(2):163–188.
- Klots EB. Odonata or dragonflies of Porto Rico and the Virgin Islands. New York Acad Sci, Survey Porto Rico. 1932;14:1–107.
- García-Díaz J. An ecological survey of the freshwater insects of Puerto Rico 1. The Odonata: with new life-histories. J Agric Univ Puerto Rico. 1938;22(1):43–97.
- Calvão LB, Vital MVC, Juen L, et al. Thermoregulation and microhabitat choice in Erythrodiplax latimaculata RIS males (Anisoptera: libellulidae). Odonatologica. 2013;42(2):97–108.
- Ball-Damerow JE, M’Gonigle LK, Resh VH. Changes in occurrence, richness, and biological traits of dragonflies and damselflies (Odonata) in California and Nevada over the past century. Biodivers Conservat. 2014;23(8):2107–2126.
- Maldonado-Benitez N, Mariani-Ríos, A, and Ramírez, A. Effects of urbanization on Odonata assemblages in tropical island streams in San Juan, Puerto Rico International Journal of Odonatology.2022,25,31–42 doi:10.48156/1388.2022.1917163.
- Flowers RW, and Pringle CM Yearly fluctuations in the mayfly (Ephemeroptera) community of a tropical stream draining lowland pasture in Costa Rica . . Current directions in research on Ephemeroptera (Canadian Scholars Press). 1995;131–150.
- Alves-Martins F, Del-Claro K, Jacobucci GB. Sexual size dimorphism, mating system and seasonality of a Neotropical damselfly, Telebasis carmesina (Coenagrionidae). Int J Odonatol. 2012;15(4):263–273.
- Ackerman J, and Galloway TD (2003). Odonata larvae in urban retention ponds in Winnipeg, Manitoba, Canada Proceedings of the Entomological Society of Manitoba. 59, 5–15.
- Hofmann TA, Mason CF. Habitat characteristics and the distribution of Odonata in a lowland river catchment in eastern England. Hydrobiologia. 2005;539(1):137–147.
- Remsburg AJ, Turner MG. Aquatic and terrestrial drivers of dragonfly (Odonata) assemblages within and among north-temperate lakes. J North Am Benthological Soc. 2009;28(1):44–56.
- Cardona-Rivera GA, Ramírez A. Predation of Telebasis vulnerata (Odonata: coenagrionidae) eggs by detritivorous caddisfly larva, Phylloicus pulchrus (Trichoptera: calamoceratidae). Int J Odonatol. 2016;19(4):253–256.
- Corbet PS. Biology of Odonata. Annu Rev Entomol. 1980;25(1):189–217.
- Pritchard G, Pelchat B. Larval growth and development of Argia vivida (Odonata: coenagrionidae) in warm sulphur pools at Banff, Alberta. Can Entomol. 1977;109(12):1563–1570.
- Fincke OM, Hedström I. Differences in forest use and colonization by Neotropical tree‐hole damselflies (Odonata: pseudostigmatidae): implications for forest conversion. Stud Neotropical Fauna Environ. 2008;43(1):35–45.
- Sánchez-Herrera M, Realpe E. Population structure of Polythore procera at a Colombian stream (Odonata: polythoridae). Int J Odonatol. 2010;13(1):27–37.
