ABSTRACT
The Brazilian Atlantic Forest (BAF) is hotspot for conservation priorities due to the high concentration and maintenance of biodiversity, comprising more than 600 species of amphibians, of which 88% are endemic to this biome. Many of these species are under some level of threat, especially those with restricted distributions to mountainous regions. Hylodes is the most speciose genus within Hylodidae, housing 26 recognized species of the diurnal frogs related to streams of forest massifs in the BAF. Hylodes pipilans is endemic to the state of Rio de Janeiro, considered restricted to the Serra do Órgãos mountains. Here, we updated its records based on fieldwork, bioacoustics analysis, and museum data, enhancing the species distribution comprehension. Additionally, we evaluate its occurrence inside of protected areas, updating information to subsidize conservation actions for this poorly known Torrent frog. We recorded individuals of H. pipilans in Parque Nacional da Serra dos Órgãos and Reserva Biológica do Tinguá. The vocalizations analyzed were compatible with the bioacoustics traits of H. pipilans. Its distribution covers part of six municipalities in the Serra dos Órgãos Mountain range, northern portion of Serra do Mar, between 245 and 814 meters above sea level. The new record to the Reserva Biológica do Tinguá is the species southernmost record. Despite the previous assessment on the conservation status of H. pipilans suggest as a least concern species, little is known about its occurrence extension, occupancy area, population trends, and ecological information. Thus, the present study is particularly important to enhance the understanding of altitudinal and geographic distribution range for H. pipilans. These will assist in the species conservation status reassessment.
Brazil comprises 14% of amphibian species of the world with 1,136 species [Citation1,Citation2]. The Neotropical Brazilian Atlantic Forest (BAF) is one of the biodiversity hotspots [Citation3] being reduced into small-disrupted fragments totaling 12% of its original area [Citation4] but still harboring more than 600 species of amphibians, of which around 80% are endemic to this biome [Citation5, Citation6]. Therefore, many species are under some level of threat, even those categorized as Least Concern (LC) are restrictedly distributed and associated with mountains tops, or mountainous complexes [Citation7–9]. This occurs due to the little knowledge concerning its occurrence extension, occupancy area, population trends, and ecological information. The lack of species distribution data is the main problem related to public policy implementation and conservation programs for amphibians [Citation10], especially in regions suffering severe habitat destruction [Citation11, Citation12].
The Torrent frog Hylodes Fitzinger 1826 is an endemic BAF genus, and the most speciose within Hylodidae Family, housing 26 recognized species [Citation2]. Allocated into four taxonomic groups, the Hylodes lateristrigatus group is the most abundant with 19 species [Citation2, Citation13]. Among these species, four might be distinguishable by having nuptial tubercles on the thumb of males: Hylodes phyllodes Heyer and Cocroft, 1986, H. fredi Canedo and Pombal, 2007, H. pipilans Canedo and Pombal, 2007, and H. caete Malagoli, Sá, Canedo and Haddad, 2017. They are distributed in the Serra do Mar Mountain range from the state of São Paulo to the central region of state of Rio de Janeiro, southeastern Brazil [Citation14].
Nowadays Hylodes pipilans is known from near to Soberbo River at Serra dos Órgãos, municipality of Guapimirim (type locality) [Citation15], from the Reserva Ecológica de Guapiaçu, municipality of Cachoeiras de Macacu [Citation16], and Parque Natural Municipal da Taquara, municipality of Duque de Caxias [Citation17], state of Rio de Janeiro, Brazil. Due to the insufficient data related to its geographic distribution, population abundance ecological and natural history [Citation15–18], this species was considered Data Deficient (DD) according to International Union for Conservation of Nature (IUCN) criteria [Citation19] and more recently it was classified as LC by the Brazilian Red List of threatened species [Citation20]. Here, we reported new records of H. pipilans within Serra do Mar, BAF, state of Rio de Janeiro, southeastern Brazil, enhancing species distribution comprehension. Additionally, we evaluate its occurrence inside of protected areas updating its data to subsidize conservation actions for this poorly known torrent frog.
We conducted visual encounter surveys [Citation21] for amphibian sampling in two protected areas including in Category II of the IUCN [see [Citation22]]: Parque Nacional da Serra dos Órgãos (hereafter PARNASO; 22°34ʹ22.1” S; 43°9ʹ49.7” W) and Reserva Biológica do Tinguá (hereafter REBIO Tinguá; 22°34ʹ34.00” S; 43°27ʹ52.40” W), both located in the central region of the state of Rio de Janeiro, southeastern Brazil. We observed the resting and vocalization sites of the species and the streams width was measured using a tape measure. Collected specimens were identified as H. pipilans according morphological and bioacoustics characters described by Canedo and Pombal [Citation15]. Calls of two recorded males were used for the comparisons. Bioacoustics analyses and its relative figures were performed using Raven Pro 1.5 software from the Cornell Laboratory of Ornithology [Citation23]. Spectrograms were produced using a window function Hann, 1024 point DFT size, 99% time grid overlap, filter bandwidth (Hz) 135, brightness 55% and contrast 60%. For description of the calls we followed the same terminology used by Canedo and Pombal [Citation15] (note-centered terminology; sensu Köhler et al. [Citation24]). Quantitative parameters are summarized in and are shown as ranges [Minimum (Min) – Maximum (Max)] followed by Mean (x) ± Standard Deviation (SD), and sample size (N) [Min – Max (x ± SD, N)]. Voucher specimens were collected, anesthetized, and killed with lidocaine 2%, fixed with formaldehyde 10% for posterior preservation in 70% alcohol, and deposited in the Museu Nacional, Universidade Federal do Rio de Janeiro (MNRJ). The Sound files are deposited in the audio collection (MNVOC) of the same institution and also available at the Fonoteca Neotropical Jacques Vielliard (FNJV). Additional data were obtained from analyzed specimens of Hylodes pipilans deposited in the museum collections of MNRJ and Amphibian Collection of the Departamento de Zoologia, Universidade Federal do Rio de Janeiro (ZUFRJ). A geographic range map was generated in QGIS 2.6 software and coordinates expressed in WGS 84.
Table 1. Records of Hylodes pipilans for the state of Rio de Janeiro, southeastern Brazil. Elevation is provided in meters above sea level. * = outside of protected area
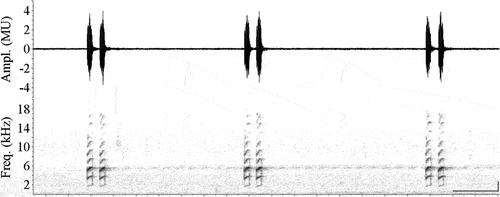
We recorded a total of 20 individuals of H. pipilans, of which 18 were recorded in the PARNASO () and two in the REBIO Tinguá. Calling males were observed in PARNASO from January to March and from October to December 2019 at 245 to 311 m above sea level (a.s.l.), in the municipality of Magé. These males were vocalizing on stones associated with permanent streams with about 2 to 4 m of width, with lower abundance in October (N = 1) and higher in December (N = 9). We analyzed 50 calls (100 notes) of two males, where only one type of acoustic signal was emitted (). This call was classified as advertisement call and clearly refers to Hylodes pipilans (see [Citation15] and listen to FNJV 45920). The advertisement call of H. pipilans from PARNASO (municipality of Magé) consists of two short notes with duration of 0.07–0.09 s (0.08 ± 0.002; N = 50 calls), note repetition rate of 23.87–26.79 notes/s (25.42 ± 0.617; N = 100) and inter-call intervals of 0.566–1.4 s (0.82 ± 0.172; N = 48). The inter-note intervals varied between 0.075 and 0.084 s (0.079 ± 0.002, N = 49), with each note during of 0.020–0.028 s (0.024 ± 0.002; N = 100). Harmonics are visible in the spectrogram. The dominant frequency (third harmonic) of first note varied of 5156.2–5625 (5466.2 ± 111.72; N = 50) and of second note varied of 4968.8–5531.2 (5377.5 ± 129.52; N = 50).
Figure 1. (A) Voucher specimen of Hylodes pipilans (MNRJ 92155) collected in the municipality of Magé, state of Rio de Janeiro, southeastern Brazil. Aspects of breeding sites in the (B) municipality of Magé (PARNASO) and (C) Nova Iguaçu (REBIO Tinguá).

Figure 2. Advertisement call of Hylodes pipilans (MNRJ 92155) from the Parque Nacional da Serra dos Órgãos, municipality of Magé, state of Rio de Janeiro, southeastern Brazil. Oscilogram and spectrogram showing three calls emitted in sequence. Note the presence of a high number of highlighted harmonics (ca. ten harmonics). Horizontal scale bars have 0.2 s; vertical scale bars have 1 kHz. Filter bandwidth (Hz): 135.
Individuals from REBIO Tinguá in the municipality of Nova Iguaçu were observed in August 2018 and July 2020. These individuals were captured during nighttime surveys around 700 m a.s.l. Both males were found at rest, under rocks on the margin of a stream with about 1 m of width. Vocalizing males were not observed during the day’s surveys. This is the southernmost locality of the species. Based on the species’ original description [Citation15] and analyzed specimens housed in the amphibian collection of MNRJ, we concluded that some individuals of H. charadranaetes from the municipality of Saquarema, state of Rio de Janeiro, were mistakenly identified as H. cf. pipilans [Citation25]. Thus, the record for the Reserva Biológica de Guapiaçu, in the municipality of Cachoeira de Macacu, is the northmost record for the species.
Amphibians are among the most threatened vertebrate group, with approximately one-third of the species under threat of extinction [Citation26, Citation27], also have the highest percentage of species remaining completely outside of protected areas [Citation12, Citation28]. Associated with the aforementioned information, the lack of knowledge about the different biological aspects of amphibian species may place them at a higher extinction risk than is currently recognized [Citation29]. Regarding species of Hylodes, nine of the 25 species evaluated (except H. caete) by the Brazilian Red List of threatened species [Citation20] are categorized as DD. For the other species categorized as LC, such as H. pipilans, further investigations are necessary to get a better basis for the establishment of this categorization, since there are few criteria used for them not to be within some level of threat. This scarcity of species data associated with the attempts of expanding and/or filling the species distribution reminds us of the urgency in monitoring and mapping the poorly known species to clarify the awareness about the most varied aspects of biodiversity [Citation30, Citation31]. Hylodes pipilans has the geographical and altitudinal distribution updated for two municipalities of the state of Rio de Janeiro, southeastern Brazil: Municipality of Nova Iguaçu, in the REBIO and municipality of Magé, in the PARNASO. Both protected areas are between the largest remnants of Atlantic Forest in the state of Rio de Janeiro. Herein, we enlarge the acquaintance about the pattern of distribution of H. pipilans, which remains until now restricted to the Serra dos Órgãos mountains. The record of the species to the municipality of Magé adds its occurrence to another municipality that comprise the PARNASO (Guapimirim, Magé, Petrópolis and Teresópolis). Additionally, the record of the H. pipilans for the municipality of Magé represents the lowest altitudinal grade of occurrence reported for the species (245 m a.s.l). Vocalizing males of this sample site were recorded for comparison with the previous description of this species of call. According to Canedo and Pombal [Citation15] and confirmed by our analysis, the advertisement call of Hylodes pipilans can be distinguished from the calls of all species of Hylodes phyllodes group by its number of notes per call (consists of two notes). Furthermore, others analyses quantitative parameters had little variation among the samples, such as duration of call, note repetition rate, note duration and dominant frequency. Thus, we are confident that the bioacoustics analyzes presented in this study facilitated the identification of the species in the municipality of Magé. In addition, the record of species to the REBIO Tinguá extends its distribution 78 km southwest of the type locality and constitutes the southernmost record for the species, with approximately 25 km from nearest previously known distribution. Of the nine localities where H. pipilans was recorded, only one is outside of protected area (). Evaluation of patterns of species distribution that exhibit low environmental plasticity is required from the conservation viewpoint [Citation32, Citation33]. Although some Hylodes species have restricted distributions to elevations above 800 meters (e.g. H. japi and H. sazimai [Citation34, Citation35]), species with distribution associated to the Serra do Mar mountain range, whose males have nuptial thumb tubercles, seem to have more variable elevational distributions. Hylodes phyllodes is widely distributed, occurring along a variable elevational gradient (50–1100 meters; L. Malagoli pers. obs.), whereas H. caete seems restricted to medium and high elevations (450–900 [Citation14]), as well as H. pipilans apparently also restricted to medium elevational range (245–814). Such studies provide essential information to designate priority areas for conservation, as well as to determine species extinction vulnerability [Citation36]. Hylodes pipilans require attention concerning their conservation due to their high habitat specificity (stones associated with permanent streams) and restricted distribution (Serra do Órgãos mountains). Some Hylodes species were reported by having chytridiomycosis, a highly virulent disease caused by the fungus Batrachochytrium dendrobatidis [Citation36–38]. The modification habitats and emerging infectious diseases may accelerate the population declines of the species that, such as H. pipilans, are closely associated with streams in forested areas [Citation39]. Therefore, data about its distribution and ecological parameters are necessary to ascertain its conservation status.
As well as the IUCN Red List criteria for species [Citation40], the Brazilian Red List of threatened species uses several objective and quantitative criteria to categorize the species conservation status on a global scale [Citation41]. The first one is the range of current species distribution and how these geographical areas have been affected by anthropogenic actions. Hence, an accurate categorization of the conservation status relies on the species geographical distribution assessment. Finding new occurrence records can contribute to reduce the chance of a species to be included in a threat category during an assessment [Citation42]. Thus, the present study is particularly important to enhance the understanding of altitudinal and geographic distribution range for H. pipilans.
Acknowledgments
We are indebted to U. Caramaschi, J.P. Pombal Jr., M. Woitovicz-Cardoso and P. Pinna (MNRJ, UFRJ) for allowing the use of specimens under their care. The specimens were collected under permit #62056-5 and #62282, granted by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). LFC (132763/2020-6) and LRM (141259/2014-0) thank both the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for supporting. LRM (#2014/23677-9) thanks to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Segalla MV, Caramaschi U, Cruz CAG, et al. Brazilian amphibians: list of species. Herpetol bras. 2016;8:65–96.
- Frost DR 2020. Amphibian species of the world: an online reference. Version 6.0. American Museum of Natural History, New York USA. Available: http://research.amnh.org/vz/herpetology/amphibia/ [cited 2020 Oct 9].
- Myers N, Mittermeier RA, Mittermeier CG, et al. Biodiversity hotspots for conservation priorities. Nature. 2000;403(6772):853–858.
- Ribeiro MC, Metzger JP, Martensen AC, et al. The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? implications for conservation. Biol Conserv. 2009;142(6):1141–1153.
- Haddad CFB, Toledo LF, Prado CPA, et al., Guia dos anfíbios da Mata Atlântica - Diversidade e Biologia. Anolis Books Editora. São Paulo. 2013.
- Rosa-Feres DC, Garey MV, Caramaschi U, et al. Anfíbios da Mata Atlântica: lista de espécies, histórico dos estudos, biologia e conservação. Monteiro-Filho ELA, Conte CE, editors. Revisões em zoologia: mata Atlântica. 1. ed. Curitiba:Ed. UFPR; 2017. p.237–314.
- Lemes P, Melo AS, Loyola RD. Climate change threatens protected areas of the Atlantic Forest. Biodivers Conserv. 2014;23(2):357–368.
- Loyola RD, Lemes P, Brum FT, et al. Clade-specific consequences of climate change to amphibians in Atlantic Forest protected areas. Ecography. 2014;37(1):65–72.
- Vasconcelos TS, Nascimento BTM, Prado VHM. Expected impacts of climate change threaten the anuran diversity in the Brazilian hotspots. Ecol Evol. 2018;8(16):7894–7906
- Young B, Stuart SN, Chanson JS, et al. Disappearing jewels: the status of new world amphibians. Arlington: NatureServe; 2004.
- Becker CG, Fonseca CR, Haddad CFB, et al. Habitat split and the global decline of amphibians. Science. 2007;318(5857):1775–1777.
- Guerra V, Jardim L, Llusia D, et al. Knowledge status and trends in description of amphibian species in Brazil. Ecol Indic. 2020;118:106754.
- Heyer WR. Two new species of the frog genus Hylodes from Caparaó, Minas Gerais, Brasil (Amphibia: leptodactylidae). Proc Biol Soc Wash. 1982;95:377–385.
- Malagoli LR, FP S, Canedo C, et al. A new species of Hylodes (Anura, Hylodidae) from Serra Do Mar, Southeastern Brazil: the fourth with nuptial thumb tubercles. Herpetologica. 2017;73(2):136–147.
- Canedo C, Pombal-Jr JP. Two new species of torrent frog of the genus Hylodes (Anura, Hylodidae) with nuptial thumb tubercles. Herpetologica. 2007;63(2):224–235.
- Silva-Soares T, Weber LN, Salles ROL. Amphibia, Anura, Hylodidae, Hylodes pipilans: distribution extension. Checklist. 2008;4(3):295–296.
- Salles ROL, Weber LN, Silva-Soares T. Amphibia, Anura, Parque Natural Municipal da Taquara, municipality of Duque de Caxias, state of Rio de Janeiro, southeastern Brazil. Checklist. 2009;5(4):840–854.
- Nogueira-Costa P, Weber LN, Wogel H, et al. A Description of the Tadpoles of Hylodes pipilans Canedo and Pombal, 2007: an Endemic Species of the Atlantic Forest of Brazil. Herpetol Conserv Bio. 2019;14(2):370–379.
- Angulo A. Hylodes pipilans. The IUCN red list of threatened species 2008. [updated 2008 January 01; cited 2021 April 18]. Available from. 2008; 10.2305/IUCN.UK.2008.RLTS.T135733A4194607.en
- Instituto Chico Mendes de Conservação da Biodiversidade. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção, ICMBio, Brasília, Brasil. [updated 2018; cited 2021 October 06]. Available from: https://www.icmbio.gov.br/portal/images/stories/comunicacao/publicacoes/publicacoes-diversas/livro_vermelho_2018_vol1.pdf.
- Crump ML, Scott-Jr NJ . Visual encounter surveys. In: Heyer WR editor. Measuring and Monitoring Biological Diversity. Standard Methods for Amphibians. Washington (DC): Smithsonian Institution Press; 1994. p. 84–92.
- Dudley N. Guidelines for applying protected area management categories. International Union for the Conservation of Nature (IUCN). Gland: Switzerland [updated 2008; cited 2021 October 06]. Available from: https://portals.iucn.org/library/sites/library/files/documents/PAG-021.pdf.
- Bioacoustics Research Program. Raven Pro: Interactive Sound Analysis Software. Version 1.5.; 2019; Cornell Lab of Ornithology, New York. Available from: http://www.birds.cornell.edu/brp/raven/RavenOverview.html.
- Köhler J, Jansen M, Rodríguez A, et al. The use of bioacoustics in anuran taxonomy: theory, terminology, methods and recommendations for best practice. Zootaxa. 2017;4251(1):1–124.
- Martins A, Pontes R, Mattedi C, et al. Herpetofauna community from coastal restinga remnants in Northeast Rio de Janeiro state, Brazil. J Coast Conserv. 2019;23(6):1019–1037
- International Union for Conservation of Nature – IUCN. 2017. The IUCN Red List of threatened species. [updated 2021-2; cited 2021-2; cited 2021 May 26]. Available from: http://www.iucnredlist.org.
- Stuart SN, Chanson JS, Cox NA, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306(5702):1783–1786.
- Roll U, Feldman A, Novosolov M, et al. The global distribution of tetrapods reveals a need for targeted reptile conservation. Nat Ecol Evol. 2017;1(11):1677–1682.
- Howard SD, Bickford DP. Amphibians over the edge: silent extinction risk of data deficient species. Divers Distrib. 2014;20(7):837–846.
- Haddad CFB. Uma análise da lista brasileira de anfíbios ameaçados de extinção. Livro Vermelho da fauna brasileira ameaçada de extinção. 2008;2:287–295.
- Hortal J, Bello F, Diniz-Filho JAF, et al. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu Rev Ecol Evol. 2015;46(1):523–549
- Giovanelli JGR, Araujo CO, Hadadand CF, et al. Ecological modelling of Phyllomedusa ayeaye (Anura: hylidae): prediction of new occurrence areas for a rare species. Neotrop Biol Conserv. 2008;3:59–65.
- Santa-Cruz R, Delgado WL, Medina CE, et al. Distribution and conservation status of the critically endangered harlequin frog Atelopus epikeisthos (Anura: bufonidae). Salamandra. 2017;53(3):423–425.
- de Sá FP, Canedo C, Lyra ML, et al. A new species of Hylodes (Anura, Hylodidae) and its secretive underwater breeding behavior. Herpetologica. 2015;71(1):58–71.
- Vidigal I, Montesinos R, Giaretta AA. A genetic and acoustic evaluation of the distribution of Hylodes sazimai Haddad & Pombal, 1995 (Hylodidae), a stream-dwelling Atlantic Forest frog. J Herpetol. 2015;55:253–264.
- Toledo LF, Brito FB, Araújo OGS, et al. The occurrence of Batrachochytrium dendrobatidis in Brazil and the inclusion of 17 new cases of infection. S Am J Herpetol. 2006;1(3):185–191.
- Toledo LFB, Haddad CF, Carnaval ACOQ, et al. A Brazilian anuran (Hylodes magalhaesi: leptodactylidae) infected by Batrachochytrium dendrobatidis: a conservation concern. Amphib Reptile Conserv. 2006;4:17–21.
- Vieira CA, Toledo LF, Longcore JJ, et al. Body length of Hylodes cf. ornatus and Lithobates catesbeianus tadpoles, depigmentation of mouthparts and presence of Batrachochytrium dendrobatidis are related. Braz J Biol. 2013;73(1):195–199.
- Almeida–Gomes M, Lorini ML, Rocha CFD, et al. Underestimation of extinction threat to stream–dwelling amphibians due to lack of consideration of narrow area of occupancy. Conserv Biol. 2014;28(2):616–619.
- International Union for Conservation of Nature – IUCN. The IUCN Red List Categories and Criteria. Version 3.1. Gland; Switzerland; Cambridge: IUCN. IUCN Species Survival Commission; 2001.
- Brito D, Ambal RG, Brooks T, et al. How similar are national red lists and the IUCN red list? Biol Conserv. 2010;143(5):1154–1158.
- Paglia AP, Fonseca GAB. Assessing changes in the conservation status of threatened Brazilian vertebrates. Biodivers Conserv. 2009;18(13):3563–3577.
- Siqueira CC, Vrcibradic D, Rocha CFD. Altitudinal records of data deficient and threatened frog species from the Atlantic Rainforest of the Serra dos Órgãos, in southeastern Brazil. Braz J Biol. 2013;73(1):229–230.
