ABSTRACT
Parastacus pugnax is an endemic burrowing crayfish from the basins of the Mediterranean and Temperate Wet regions of Chile. For the first time, specimens were found incidentally in the Semiarid Region of Chile (Choapa River Basin). In these regions, hydrological changes derived from global warming and the intensive use of water for irrigation have resulted in highly fragmented habitats, where the effects of the dry season are exacerbated by periods of permanent drought. Unfortunately, in the Semiarid Region there are not regulatory measures for the conservation of the native biota. The aims of this study were to determine the spatial distribution patterns of P. pugnax in the Choapa River Basin (⁓31°S) to expand and contribute with updated knowledge about the distribution and natural history of this species highlighting local environmental management issues. Seasonal occurrence surveys were carried out at eight sites stratified by elevation in the lower, middle, and upper zones of this basin. This was complemented by semi-structured interviews within local prawn´s fisher. Parastacus pugnax was distributed across ⁓25 km in the lower and middle zone of the basin, and it is present in lentic and lotic environments. The occurrence of this excavator species in lotic environments is somehow unusual according to its ecology, which suggests that it is a primary facultative excavator, so a re-evaluation of its current ecological category (category 1; primary excavator) is necessary. According to the interviewees, P. pugnax corresponds to a naturalized species introduced by humans ⁓25–30 years ago for artisanal aquaculture in ponds. The wide altitudinal distribution of P. pugnax in the Choapa River Basin indicates the need to re-evaluate its distribution limits, which traditionally placed the Parastacidae family to the south of Aconcagua River Basin (⁓33°S). To understand the origin of the species in the Choapa River Basin, is clue to specify the phylogenetic structure and determinate whether the presence in the Semiarid Region is constrained to the Choapa River Basin or extends to other basins within this region.
Introduction
The geographic range of a species corresponds to a representation of the spatial distribution, which is determined by the biological limits for its existence in a given habitat [Citation1]. Although the geographic distribution is the main axis of biogeography, the knowledge of the species range is a basic question for planning conservation strategies and management of biological diversity. This is even more relevant in the case of biodiversity of inland waters, since these types of ecosystems are particularly vulnerable to anthropogenic activities both in Chile [Citation2] and globally [Citation3].
The current geographical configuration of continental Chile implies low or scarce connectivity among hydrographic units, thus developing a high degree of endemism of the faunal components that inhabit there. This aspect is accentuated in species with low vagility or those species whose habitat requirements are highly specific, such as bivalves, gastropods, and malacostraceans [Citation4]. In this last case, one species of prawn and seven species of crayfishes inhabit the continental waters of Chile, belonging to the families Palaemonidae (Cryphiops caementarius (Molina, 1782)) and Parastacidae (Samastacus spinifrons (Philippi, 1882), Parastacus pugnax (Poeppig, 1835), Parastacus nicoleti (Philippi, 1882), Virilastacus araucanius (Faxon, 1914), Virilastacus rucapihuelensis Rudolph & Crandall 2005, Virilastacus retamali Rudolph & Crandall 2007, and Virilastacus jarai Rudolph & Crandall 2012) [Citation5,Citation6]. In relation to the status of conservation, the Chilean Environment Ministry (MMA) classifies these crustaceans into four categories: Least Concern (LC) (S. spinifrons, P. pugnax, P. nicoleti), Vulnerable (VU) (C. caementarius, V. araucanius), Data Deficient (DD) (V. retamali, V. jarai), and Endangered (EN) (V. rucapihuelensis). See http://www.especies.mma.gob.cl/ for more details.
The foregoing shows that the continental decapods of Chile exist under a significant level of threat, which in turn, have been poorly studied, showing significant information gaps. In particular, when evaluating the impact of anthropogenic activities on their populations. Despite this, there have been interesting findings in recent years, such as the description of new taxa of burrowing crayfishes, such as V. retamali, V. jarai and V. rucapihuelensis, whose distribution are restricted to some freshwater systems that flow through the Coastal Mountain Range of southern Chile [Citation6]. Recent phylogenetic studies on the populations of the burrowing crayfish P. pugnax, show that the lineage of this species has different demographic histories, which do not necessarily coincide with the historical processes of hydrographic basin formation in Chile [Citation7]. This background information reflects the gap of knowledge about the natural history of freshwater malacostraceans and the need for further investigation on the life history of Chilean parastacids.
Parastacus pugnax is the burrowing crayfish with the widest geographical distribution within Parastacidae family in Chile [Citation8]. The species covers a latitudinal range of ⁓700 km across the intermediate depression of Chile (The central valley between the Andes and the coastal ranges) inhabiting scattered semi-marshland lentic habitats, such as “vegas” and “hualves” [Citation9]. The species can also be found less frequently in lotic environments, such as streams and rivers [Citation10]. The latitudinal distribution range of P. pugnax covers from the Aconcagua River Basin (⁓33°S; Mediterranean Region) to the Toltén River Basin (⁓39°S; Temperate Wet Region) [sensu Citation7, Citation8, Citation9] (gray outline; ). In much of its distribution range, P. pugnax is subjected to strong extractive pressure (43.5 million of individuals year−1) for commercial purposes, especially in the south-central Chile [Citation9]. As this area concentrates the largest number of populations, consequently, it also exhibits the bulk of the auto-ecological studies [Citation9]. This contrasts with the scarcity of studies on the populations of P. pugnax in northern-central Chile. In this zone, mining and agriculture have led to permanent water depletion [Citation11] driven both by canalization of the river flow for irrigation and changes in the rainfall regime caused by global warming. This resulted in highly fragmented habitats [Citation12], which currently, do not have regulatory measures that protect the aquatic biota.
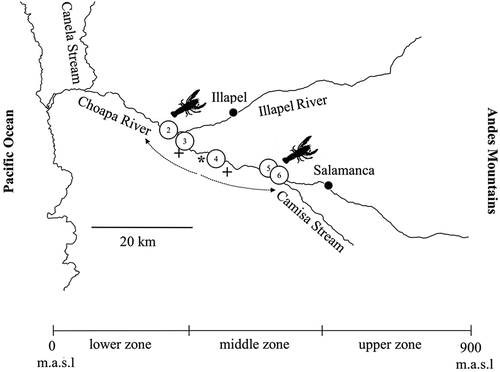
Figure 1. Geographic distribution range of Parastacus pugnax in Chile. a) The gray outline indicates the historical distribution range and the black outline indicates the study area in the Choapa River Basin, northern-central Chile. Climatic and hydrographic regions of Chile (Geographic Military Institute of Chile – IGM) [Citation14,Citation15]. b) Altitudinal stratification of the sampling sites. Ar = Arid, SAr = Semiarid, Me = Mediterranean, TW = Temperate Wet, CW = Cold Wet. * = DGA fluviometric station.
![Figure 1. Geographic distribution range of Parastacus pugnax in Chile. a) The gray outline indicates the historical distribution range and the black outline indicates the study area in the Choapa River Basin, northern-central Chile. Climatic and hydrographic regions of Chile (Geographic Military Institute of Chile – IGM) [Citation14,Citation15]. b) Altitudinal stratification of the sampling sites. Ar = Arid, SAr = Semiarid, Me = Mediterranean, TW = Temperate Wet, CW = Cold Wet. * = DGA fluviometric station.](/cms/asset/ecc44fac-b8ca-42aa-a781-6b9168558e6d/tneo_a_2049173_f0001_b.gif)
The central aims of this study were to determine the spatial distribution patterns of P. pugnax in the Choapa River Basin according the new findings and to expand the knowledge about its distribution and natural history in continental Chile. This ecological information is essential for the generation of plans and programs aiming to improve the conservation status of the unique biodiversity of the Semiarid Region [Citation13], especially in basins that are facing the worst droughts of the last decades [Citation12].
Methods
Spatial scope
The Choapa River Basin (⁓31°S) (CRB, hereafter), is located in the Semiarid Region of northern-central Chile () and is characterized by periods of prolonged droughts from summer to autumn, and a rainfall season concentrated in winter [Citation14,Citation15]. These contrasting periods generate high variability in both the monthly average flow and depth of rivers. During 2020, these parameters registered a minimum of 0.11 m3 s−1 and 0.16 m, during January (summer) respectively, and reached a maximum of 1.87 m3 s−1 and 0.57 m in July (winter), respectively (General Water Directorate of Chile – DGA; see https://dga.mop.gob.cl/). The water temperature fluctuated between 26.3°C in summer and 12.6°C, in winter [Citation13]. The river banks in the study area were dominated by the macrophytes Baccharis spp., Hydrocotyle spp., and Nasturtium officinale W.T. Aiton, and the shallow waters by Myriophyllum spp., and Ludwigia peploides (Humb. Bonpl, & Kunth) P.H. Raven. These macrophytes are well-known invasive species and indicators of eutrophic conditions, which account for a river ecosystem with a high degree of anthropogenic intervention [Citation13].
Specimen capture
During the population surveys of the freshwater prawn Cryphiops caementarius in CRB (2020–2021) carried out by the Institute of Fisheries Development (IFOP) (see https://www.ifop.cl/programa-camaron-choapa/), local prawn´s fisher organizations reported the presence of some crayfish with a morphology that differed from C. caementarius. The fishers indicated a consistent presence of these “unfamiliar” specimens in the river since the 90ʹs.
Based on the local narrative, a parallel study was carried out in conjunction with the surveys of C. caementarius [Citation13], to determine the taxonomic identity of the rare specimens and the distribution range throughout the river basin. For that, prawn´s fishers extracted specimens through manual capture, by visual inspection of the banks following 200 m transects, in parallel to the fluvial axis. A total of 39 adult´s specimens were captured and photographed assessing the length (mm) of the cephalothorax (CL; distance from tip of rostrum to mid-dorsal posterior margin of carapace) and the total length (TL; from tip of rostrum to posterior margin of telson). The specimens were later released at the site.
To determine the altitudinal distribution range of the species along the CRB, presence/absence surveys were performed during autumn, winter, and spring of 2020 and summer of 2021 at eight study sites (). The surveys were stratified in three altitudinal gradients (meter above sea level; m.a.s.l) across the basin: lower zone (⁓0–250 m.a.s.l.; sites 1, 2, 3), middle zone (⁓250–500 m.a.s.l.; sites 4, 5, 6) and upper zone (⁓500–800 m.a.s.l.; sites 7, 8) ().
Identification of specimens
The external morphological characters of the specimens found in the CRB () were contrasted with the characters described in the diagnosis for P. pugnax provided by Rudolph [Citation9], consisting mainly of the shape and size of the rostrum, shape of the cervical grooves, presence/absence of postorbital carinas, movement of P1 dactyls, presence/absence of supernumerary gonopores and phallic papillae morphology. The final identification was achieved using the keys for Chilean species of the Parastacidae family following Rudolph [Citation16].
Local ecological knowledge
In order to clarify the origin of the specimens from CRB, semi-structured interviews were conducted with key informants from two local prawn´s fisher organizations (N = 27, age range from 32 to 71 years old), from November 2020 to June 2021. The interviewees answered five questions: a) how long the species has been present in the CRB? b) in what parts of the river are they found? c) do you know the origin of this species? if so, specify, d) is the species present in other basins in northern Chile? if so, specify, and e) do both crustaceans species compete with each other? if so, specify.
Results
Identification of specimens
Short triangular rostrum (); carapace compressed laterally; V-shaped cervical groove (); postorbital carinas slightly raised, without apical spines (); voluminous P1 chelae, of similar size with subvertical movement (); pleon reduced without spines or tubercles (); male and female gonopores present in all specimens; phallic papillae formed by a fixed, slightly raised, calcified ventromedial prominence. Body colour predominantly greenish-gray (). The CL of specimens captured varied between 17.7 and 33.0 mm. However the TL varied between 27.9 and 70.7 mm, with an average of 55.6 mm (± 15.1 SD).
Update of the geographical distribution of P. pugnax in Chile
In the CRB, the altitudinal range of P. pugnax extends from the lower (sites 2, 3) to the middle zone (sites 4, 5, 6) (), throughout ⁓25 km of fluvial axis. The presence of specimens was recorded during all sampling periods (autumn, winter, and spring 2020, and summer 2021). With this study, the distribution range extends north to the Semiarid Region of Chile, ⁓130 km north to the CRB (⁓31°S) (see black outline; ). This increased its occurrence in 21 basins out of the 100 hydrographic basins from continental Chile [sensu Citation17].
Local ecological knowledge
The group interviews yielded the following results: a) All the interviewees (N = 27; 100%) indicated that P. pugnax is present in the CRB from approximately 25–30 years ago (⁓1990–95). b) All interviewees (N = 27; 100%) indicated that the specimens are found between boulders and aquatic macrophytes, including fine sediments (mud, silt, clay) from the river banks (). Some fishers (N = 9; 33.3%) indicated the presence of underground galleries of this species in two semi-marshland sectors adjacent to the CRB (; ), this was sustained by fishers during the field campaigns. c) Regarding the origin of the species, all the interviewees (N = 27; 100%) indicated that it was introduced by farmland owners who kept them in nursery ponds on land, for food and recreational use. According to this, crayfish may have escaped through irrigation channels reaching river streams and establishing themselves. Fishers (N = 6; 22.2 %), indicated that the point of introduction of P. pugnax to the river is close to site 4 () and from there, it expanded to other areas of the river. d) Regarding the presence of P. pugnax in other basins in northern Chile, all the interviewees (N = 27; 100%) indicated that it is only present in the CRB. e) In general, there is a lack of knowledge about the interspecific relationships between P. pugnax and C. caementarius, and only a third of the fishers interviewed (N = 9; 33.3%) indicated that P. pugnax has “better resistance” to periods of drought than C. caementarius due to, principally, to the burrowing capacity exhibited by P. pugnax.
Figure 4. Representative habitat of Parastacus pugnax in the Choapa River Basin. Lotic environment: a) fluvial axis tracked by prawn’s fishers. b) boulders and aquatic macrophytes, including fine sediment in the water. Lentic environment: c) semi-marshland plains bordering the fluvial axis. d) presence of “chimneys” or excavations made on mud deposits around inlets holes to underground galleries. e) inlets holes to underground galleries (white circle).

Discussion
The finding of P. pugnax in the CRB is the first official record of this species inhabiting both lotic and lentic environments in the Semiarid Region of Chile, and reflects its great adaptive capacity for areas with marked hydrological variations including unusually extended periods of drought. This suggests that the semi-terrestrial burrowing habits that characterizes this species allow them to build underground galleries up to 3 m deep [Citation18], reaching microhabitats where surface oscillations in temperature and humidity are minimized. This may be particularly relevant during the summer season, providing a safe and protected environment to develop their life cycle [Citation9].
Within its natural range, P. pugnax is a crayfish well adapted to life in semi-marshland environments, away from permanent bodies of water [Citation9]. The occurrence of this species in lotic environments, such as the Choapa River, is unusual in the context of the ecology for this species. A similar case was reported in a stream in central-southern Chile [Citation10], so the present finding adds new evidence that supports the occurrence of P. pugnax in this type of environment, raising new questions about the life history of burrowing parastacids in Chile. According to the ecological classification of burrowing crayfish proposed by Hobbs [Citation19], P. pugnax is in “category 1” or “primary excavator”, because (i) the underground galleries are far from permanent bodies of water, and (ii) the life cycle develops completely inside these galleries. This classification applies to specimens that inhabit semi-marshland environments, such as the sectors surrounding the Choapa River (see ). Specimens were also found in permanent waters, inhabiting sectors with boulders, aquatic macrophytes with sediment inclusion (see ) and interestingly, in absence of gallery inlets. Accordingly, the classification as “primary excavator” would fit better into “category 1A”, proposed by Horwitz & Richardson [Citation20], as follows: (i) occurs in surface waters, associated to rocks and submerged plant structures, (ii) inhabit simple, unbranched galleries in the substrate. So, it feels necessary to review the current ecological classification of this species, since the ecological record presented here suggests that the parastacid would be a facultative primary excavator (excavate according to local hydrological conditions).
The local evidence provided by prawn’s fishers indicates that the resident populations of P. pugnax in the lower and middle zone of the CRB correspond to naturalized populations that were introduced by human action ⁓25–30 years ago, currently, reaching a spatial distribution of ⁓25 km. Although the naturalization of P. pugnax in this basin is highly probable (as reported by fishers), the restricted distribution in the lower and middle zone suggests probably a relict population [Citation7] product of the severe habitat fragmentation that affects this area [Citation13].
\Parastacus pugnax traditionally has been located nearly the Mediterranean Basin of the Aconcagua River (⁓33°S), which marks the upper limit of distribution of the family Parastacidae from mediterranean and temperate wet basins [sensu Citation5,Citation6,Citation8,Citation18,Citation21–24]. Thus, the presents record of P. pugnax in the CRB within the Semiarid Region inhabited by C. caementarius (family Palaemonidae) evidence a spatial overlapping between both species. This suggests the reassessment of the distribution limit of P. pugnax towards the Semiarid Region of Chile.
To understand if the presence of P. pugnax in the CRB constitutes a naturalized or a relict population, at least two predictive hypotheses arise. The first, is that P. pugnax is an exotic species with unknown population status, if this were the case, it is necessary to specify the phylogenetic and zoogeographic origin of this species in the CRB. The second hypothesis is that the population of P. pugnax in the CRB could constitute a relict population after successive events of local extinction in river basins down south. The absence of P. pugnax in the basins of Conchalí, Quilimarí, Petorca, and La Ligua rivers (range between the basins of Choapa and Aconcagua rivers; ⁓31–33°S) due to the destruction of the fluvial habitat leads to hypothesize that its populations could have been eradicated. If this were the case, it is necessary to determine whether the presence of P. pugnax in the Semiarid Region of Chile is particularly constrained to the CRB or in other nearby basins.
Currently, the population of P. pugnax is at risk of extinction in the Semiarid Region of Chile, due to availability of viable lentic space to build underground galleries given the intensive use of water and the prolonged droughts that affect this area [Citation13]. All of the above reveals the existence of an important gap of knowledge that implies inadequate management of local and regional diversity in freshwater basin ecosystems. It is crucial to update the state of knowledge of the populations of P. pugnax in the Semiarid Region of Chile, clarifying their zoogeographic origin, for instance, exploring patterns of genetic differentiation. At the same time, characterizing and valuing the singularities of the arid fluvial ecosystems of temperate latitudes of South America.
Acknowledgments
We thank Denisse Torres-Avilés and Alvaro E. Wilson (IFOP, Coquimbo), and the prawn´s fisher organizations from Choapa Province for the support in the field work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Brown JH, Stevens GC, Kaufman DM. The geographic range: size, shape, boundaries, and internal structure. Annu. Rev. Ecol. Syst. 1996;27(1):597–623
- Habit E, Górski K, Alò D, et al. Biodiversidad de Ecosistemas de Agua Dulce. Santiago: Mesa Biodiversidad-Comité Científico COP25, Ministerio de Ciencia, Tecnología, Conocimiento e Innovación de Chile; 2019.
- Albert JS, Destouni G, Duke-Sylvester SM, et al. Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio. 2020;50(1):85–94.
- Corporación Nacional de Medio Ambiente. Biodiversidad de Chile: patrimonio y desafíos. Santiago: Ocho Libros Editores; 2008.
- Jara C, Rudolph EH, González E. Estado del conocimiento de los Malacostráceos dulceacuícolas de Chile. Gayana. 2006;70:40–49.
- Rudolph EH. Current state of knowledge on Virilastacus species (Crustacea, Decapoda, Parastacidae). Lat. Am. J. Aquat. Res. 2015;43(5):807–818
- Victoriano PF, D’Elía G. Evolving in Islands of mud: old and structured hidden diversity in an endemic freshwater crayfish from the Chilean hotspot. Nature. 2021;11:8573.
- Rudolph EH. Sobre la distribución geográfica de las especies chilenas de Parastacidae (Crustacea: decapoda: astacidae). BB Chile. 2010;3:32–46.
- Rudolph EH. Parastacus pugnax (Poeppig, 1835) (Crustacea, Decapoda, Parastacidae): conocimiento biologico, presion extractiva y perspectivas de cultivo. Lat. Am. J. Aquat. Res. 2013;41(4):611–632
- Rudolph EH. Unexpected discovery of Parastacus pugnax (Decapoda, Parastacidae) in a lotic environment of central-southern Chile. Crustaceana. 2012;85:1657–1664.
- Carranza DM, Varas-Belemmi K, De Veer D, et al. Socio-environmental conflicts: an underestimated threat to biodiversity conservation in Chile. Environ. Sci. Policy. 2020; 110: 46–59.
- Garreaud RD, Boisier JP, Rondanelli R, et al. The central Chile mega drought (2010–2018): a climate dynamics perspective. Int. J. Climatol. 2020;40(1):421–439.
- Velásquez C, Wilson AE, Torres-Avilés D, et al. Propuesta de plan de manejo integrado para el camarón de río del norte (Cryphiops caementarius) en la cuenca del río Choapa. Coquimbo: Instituto de Fomento Pesquero; 2022.
- Romero H. Climas. Geografía de Chile, Tomo XI. Santiago: Instituto Geográfico Militar de Chile; 1985.
- Niemeyer H, Cereceda P. Hidrografía Geografía de Chile, Tomo XI. Santiago: Instituto Geográfico Militar de Chile; 1985.
- Rudolph EH. Clave taxonómica ilustrada de las especies chilenas de Parastacidae (Crustacea, Decapoda, Astacidea). Bol. Mus. Nac. Hist. Nat. 2019;68:17–32.
- Dirección General de Aguas. Atlas del Agua, Chile 2016. Santiago: Ministerio de Obras Públicas de Chile; 2016.
- Jara C. Camarones dulceacuícolas en Chile. Valdivia: Universidad Austral de Chile, Publicaciones Ocasionales; 1994.
- Hobbs HH Jr. The crayfishes of Florida. Florida: University of Florida Publications, Biological Series; 1942.
- Horwitz PHJ, Richardson AMM. An ecological classification of the burrows of Australian freshwater crayfish. Mar. Freshw. Res. 1986;37(2):237–242
- Rudolph EH. Freshwater malacostraceans in Chilean inland waters: a checklist of the Chilean Parastacidae (Decapoda, Astacidea). Crustaceana. 2013;86(12):1468–1510.
- Velásquez C, Henríquez-Antipa L, Torres-Avilés D, et al. Knowledge status of predators of the freshwater prawn Cryphiops caementarius (Decapoda: palaemonidae) in river systems along the North Western Andean region from Perú and Chile. Rev. Biol. Trop. 2020;68(4):1062–1072.
- Bahamonde N, López MT. Decápodos de aguas continentales en Chile. Invest. Zool. Chil. 1963;10:123–149.
- Bahamonde N, Carvacho A, Jara C, et al. Categorías de conservación de decápodos nativos de aguas continentales de Chile. Bol. Mus. Nac. Hist. Nat. 1998;47. 91–100.