ABSTRACT
The upper Jejuí River is a major tributary of the Paraguay River in eastern Paraguay and is the principal drainage for the Mbaracayú Forest Biosphere Reserve. Despite the international significance of the biosphere reserve and detailed documentation of its terrestrial fauna and flora, the fish fauna in its streams remains relatively unknown, with 48 species previously recorded. We sampled 35 sites within the biosphere reserve over five years (2007–2011) to assess the richness of its ichthyofauna. These surveys yielded a new total of 105 known species for the biosphere reserve, including two newly-recorded orders, Gymnotiformes and Cyprinodontiformes, and 14 newly-recorded families. Fish community composition resembled that of previously-reported fish communities from the Paraguay River drainage, with characiforms and siluriforms comprising the majority of the species (57.1% and 31.4%, respectively). Post hoc analyses showed significantly greater ichthyofaunal diversity at sites within the Mbaracayú Forest Nature Reserve, a core protected forest area within the biosphere reserve, compared to areas outside the nature reserve, suggesting a negative impact from deforestation and land conversion on fish assemblages in these headwater streams. Broad regional deforestation places greater emphasis on important management decisions that will protect current biodiversity.
Introduction
The diversity of fishes in the Neotropical biogeographic region, with greater than 6,000 described species, surpasses that of other biogeographic regions [Citation1]. Currently, this diversity is threatened by numerous anthropogenic activities [Citation2] that impact rivers and streams both directly (e.g. dams and channelization) and indirectly (e.g. agriculture and urbanization) [Citation3]. Moreover, it is argued that freshwater fishes are inadequately protected throughout the Neotropical region [Citation4,Citation5]. Freshwater ecosystems often receive ancillary conservation benefit only from the creation of terrestrial-focused protected areas [Citation6,Citation7]. Establishment of protected areas has become an increasing priority for many government and non-government entities. One such entity is the Mbaracayú Forest Biosphere Reserve (hereafter biosphere reserve) which lies within the Upper Paraná Atlantic Forest in eastern Paraguay. The biosphere reserve occurs entirely within the Jejuí River watershed, streams of which are left margin tributaries of the Paraguay River, and features the Mbaracayú Forest Nature Reserve (hereafter nature reserve) as its core protected area.
The Upper Paraná Atlantic Forest (locally referred to as the Bosque Atlántico del Alto Paraná (hereafter BAAPA) [Citation8]) is part of the Paraguay-Paraná Complex aquatic ecoregion [Citation9] and the Paraguay Freshwater Ecoregion [Citation10]. It is estimated that, as recently as 1940, the BAAPA totaled almost 9 million hectares [Citation11]. In the 1990ʹs and early 2000ʹs this ecoregion experienced some of the highest deforestation rates in the world [Citation12]. As a result, by 2000, only about 20% of the original Atlantic Forest in Paraguay remained [Citation13], which declined to less than 10% by 2016 [Citation14]. Canindeyú Department, where the biosphere reserve is located, experienced a 32.8% forest loss between 1999 and 2016 [Citation14]. A 5-km buffer zone area surrounding the biosphere reserve experienced a 39% loss of forest cover between 1989 and 2000 [Citation13].
Paraguay’s deforestation rate remains one of the highest in Latin America [Citation15–17]. Most habitat loss comes from conversion of forest to agricultural land for soybean production, cattle grazing, and subsistence farming [Citation18,Citation19]. BAAPA has been rapidly and extensively fragmented due to changing land use patterns, infrastructure development in the form of roads and dams, and unsustainable exploitation of timber for construction, furniture, and firewood [Citation8]. In addition to fragmenting the terrestrial landscape, these activities can negatively impact stream hydraulics, water quantity, water quality (via soil erosion and agrochemical pollution), and canopy cover (which raises water temperature and decreases leaf litter and woody debris) [Citation20,Citation21]. Thus, the biosphere reserve is an important remnant of BAAPA that helps protect both terrestrial and aquatic environments.
Despite a reasonably well-documented terrestrial vertebrate fauna for the biosphere reserve [Citation22] and BAAPA [Citation23–26], there exists wide variation among estimates of aquatic vertebrate diversity, and more intensive sampling of headwaters in the Paraguay River Basin has been recommended [Citation27]. Neotropical fish assemblages are often speciose and are typically dominated by characiforms and siluriforms [Citation26,Citation28–33]. Estimates as high as 450 fish species have been proposed for Paraguay based on its geographic position and resulting variety of intersecting habitats [Citation34].
Diversity of fishes in the Paraguay Freshwater Ecoregion is strongly influenced by the unique evolutionary history experienced by Neotropical fishes that included periodic interbasin exchanges between the Amazon and La Plata, the latter of which includes the Paraguay River and its tributaries [Citation28]. Additionally, given that habitat diversity promotes species diversity [Citation35–38], the numerous aquatic habitats found within the Paraguay Freshwater Ecoregion is a strong predictor of high fish diversity. Modest estimates for the Paraguay Freshwater Ecoregion, which includes all of eastern Paraguay but does not include the western Chaco region, suggest 333 species, of which approximately 35% are considered endemic [Citation39,Citation40]. Numerous reports of Paraguay fish diversity emphasize the taxonomic dominance of Characiformes and Siluriformes [Citation41–47].
As of 2005, the list of fishes known for the nature reserve included 48 species with 19 identified to genus only [Citation22]. This list was consistent with results from Paraguay fish surveys by Chernoff et al. (Paraguay River basin only) [Citation42] and Ramlow (a country-wide inventory) [Citation44] in that 56% of species presented in the nature reserve management plan were characiforms and 29% were siluriforms [Citation22]. Despite somewhat thorough survey treatments for mammals, birds, and plants, there existed only sporadic surveys of aquatic organisms in waterbodies near the reserve headquarters [Citation22]. The lack of specific identifications (some fish records for the nature reserve identified only genus) coupled with the wide range of aquatic environments within the nature reserve and broader biosphere reserve suggested that species richness in the area could be higher. Therefore, we aimed to document fish diversity in the headwaters of the upper Jejuí River in the biosphere reserve by conducting an ichthyofaunal survey. Subsequently, we used post-hoc tests to assess patterns that appeared to emerge from our survey results.
Materials and methods
Study area
The Mbaracayú Forest Nature Reserve was recognized in 1991 by Paraguay Law No. 112/91 as a joint ecosystem management effort between the Fundación Moisés Bertoni (FMB; a Paraguayan conservation non-government organization) and The Nature Conservancy [Citation22]. Initially 57,715 hectares of Atlantic Forest habitat, over the next 5 years additional and mostly Cerrado (savannah) habitat was added to create what is now a 64,405-hectare nature preserve, one of the largest protected areas in Paraguay [Citation22]. The terrestrial composition of the reserve is a habitat mosaic of 19 natural communities [Citation22]. The Mbaracayú Forest Biosphere Reserve was included in the world network of biosphere reserves in 2000 by the United Nations Educational, Scientific and Cultural Organization (UNESCO) through the Man and the Biosphere Programme (MAB) when an additional 280,000 hectares of buffer zone was included around the nature reserve to serve as a multiple use area and to help protect the core forested region [Citation22]. A hallmark of the biosphere reserve, and of Paraguay in general, is the large number of streams and rivers that traverse this land-locked country.
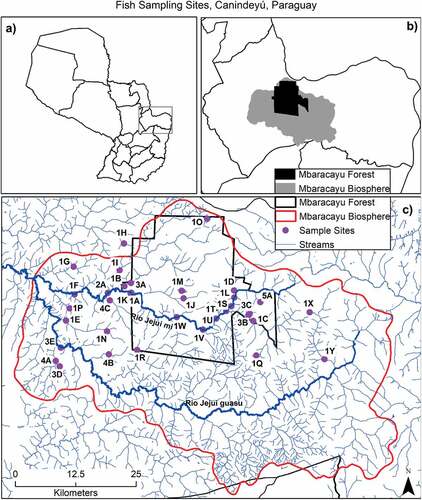
The upper Jejuí River watershed is the primary drainage of the biosphere reserve [Citation9] with two major branches, the Jejuí mi and Jejuí guasu. The Jejuí mi occurs largely within the protected core forest area (nature reserve) and the Jejuí guasu occurs largely outside the core area (biosphere reserve) ().
Figure 1. Fish sampling sites, Canindeyú, Paraguay. (A) outlines Canindeyú Department within Paraguay, (B) indicates Mbaracayú Forest Biosphere Reserve (gray) and Mbaracayú Forest Nature Reserve (black) within Canindeyú Department, and (C) the rivers and streams (in blue) in Canindeyú Department along with each sampling site (pink dots) and their reference code (see ); the Jejuí mi (northern branch) and Jejuí guasu (southern branch) rivers are indicated by thicker blue lines, the border of the biosphere reserve is indicated by the red line, and the forest reserve is indicated by the black line.
Fish sampling
We sampled 35 locations within the biosphere reserve over a 5-year period (2007–2011); 20 were within the nature reserve and 15 were outside that core forested area ( and ). Over the five years, we gathered 77 samples. We sampled 25 sites during 2007, 19 in 2008, 13 in 2009, 12 in 2010, and 8 in 2011 (). Three sites (rio Jejuí mi at the nature reserve headquarters, arroyo Guasu immediately west of the nature reserve headquarters, and arroyo Guyra Keyha at the eastern limit of the nature reserve) were sampled each year. Two sites were sampled during each of the first four years, eight were sampled three times between 2007 and 2011, eight were sampled twice, and 14 were sampled during one year only. Landowner permission was obtained prior to sampling on privately-owned land. Geographic location data were obtained using Garmin handheld GPS units (Garmin GPSMAP 60 cs). Geographic coordinates for all survey sites () were subsequently confirmed with Google Earth 7.3.2.5776 (64-bit).
Table 1.. Sampling sites in Mbaracayú Forest Biosphere Reserve, Canindeyú, Paraguay. Listings include site code (which corresponds to locations identified on ), site name, location coordinates (latitude, longitude), watershed within which the site resides, and years a sample was collected. Sites are identified by the year in which they were first sampled: sites beginning with 1 were first sampled in 2007, 2 in 2008, 3 in 2009, 4 in 2010, and 5 in 2011
Fishes were collected under research permits (28,486/07, 74,004/09, 100,188/10) granted through the Paraguay Dirección de Pesca y Acuicultura in the Dirección General de Protección y Conservación de la Biodiversidad of the Ministerio del Ambiente y Desarrollo Sostenible (DPA/DGPCB/MADES). Per agreement with the permitting authority, 50% of preserved specimens were deposited at the Museo Nacional de Historia Natural del Paraguay (MNHNP) in San Lorenzo, Paraguay. Selected specimens requiring more detailed examination were exported and are housed in the Department of Natural and Applied Sciences, University of Dubuque (Iowa, USA). Export of specimens was authorized under permit (No. 49,545) provided by DPA/DGPCB/SEAM. All remaining specimens were deposited into the scientific collection of the Jejuí mi Biological Station at the nature reserve.
The streams we surveyed presented a range of characteristics (e.g. depth, width, stream velocity, canopy cover; ). At each site, a point of entry into the river or stream formed the center of the sampling area. All sites were sampled for approximately 50 m upstream and 50 meters downstream from the entry point unless prevented by water depth or landowner boundary; in such cases, additional distance was sampled in the opposite direction to equal 100-m totals. Primary sampling methods were a straight seine (4.6 m x 1.8 m with 4.8 mm mesh) and 4.8 mm mesh dip nets (telescoping and handheld). These methods were used at all sites during all years. Minnow traps (23 cm x 42 cm) also were deployed during 2007 and 2008 at sites that could be revisited within 24 hours. In 2007, we sampled with a backpack electrofisher (Coffelt BP-4) at sites where physical conditions and accessibility allowed (n = 12), while hook-and-line sampling was utilized at five sites. In 2010, fishes also were collected from traps (81 cm x 51 cm x 30.5 cm with 6.4 mm mesh) that were used as part of a separate study on aquatic decapod crustaceans [Citation48]. Sampling occurred between May and August for 2 weeks (2009, 2011) or 8 weeks (2007, 2008, 2010).
Figure 2. Photographs of sampling sites inside the Mbaracayú Forest Nature Reserve (A and B) and outside the Mbaracayú Forest Nature Reserve, but within the Mbaracayú Forest Biopshere Reserve (C and D). Locations for each site are detailed in and indicated on . Photo A is the rio Jejuí mi at Jejuí mi (5 m width, 2 m depth, 0.6 m/sec flow; site 1A), photo B is rio Jejuí mi at Horqueta mi (2.5 m width, 1 m depth, 0.3 m/sec flow; site 1 L), photo C is rio Jejuí mi at Villa Ygatimi (10 m width, 4 m depth, 0.6 m/sec flow; site 1 F), and photo D is arroyo Moko’i (2 m width, 0.5 m depth, 0.2 m/sec flow; site 1 H).

During 2007, all fish were photographed and released; no specimens were preserved. From 2008 to 2010, in addition to photographs, all specimens were fixed in 10% formalin and then preserved in 70% ethanol. In 2011, collected fish were photographed and released with no specimens preserved.
Fishes were identified to species, when possible, through use of multiple taxonomic keys and references [Citation49–71] and compared to a comprehensive catalog of fish species for Paraguay [Citation47]. Taxonomy herein follows the Eschmeyer’s Catalog of Fishes electronic database of the California Academy of Sciences [Citation72].
All collected fishes were assigned a functional role which combined their principal adult diet with their primary foraging stratum in the water column based on their reported foraging ecology and based on prior assessments [Citation45,Citation59,Citation73,Citation74]. Fish assemblages often are categorized into functional groups, which can be defined by ecomorphology, a combination of physical features and habitat characteristics [Citation75–78]. A functional group consists of several species that perform a similar ecological role while not necessarily sharing an immediate taxonomic relationship. Thus, functional groups are defined by a shared niche rather than a shared taxonomy [Citation79]. Principal adult diets were categorized as omnivore (feeds on a wide variety of plant and animal materials), invertivore (feeds primarily on aquatic and/or terrestrial invertebrate animals), piscivore (feeds primarily on other fishes), algivore (feeds primarily on single- or multi-celled algae), or detritivore (feeds primarily by ingesting organic detritus and extracting nutrients from the material itself and any organisms residing within) [Citation52,Citation73]. Foraging strata within the water column water were defined as surface (forages primarily at the air /water interface), mesopelagic (forages primarily in the mid-section of the water column), benthopelagic (forages primarily at, or within several centimeters of, the substrate), and benthic (forages primarily on or below the substrate surface) [Citation74]. Functional diversity is defined in our study as the number of functional groups, formed from a combination of primary diet and primary water foraging stratum.
Statistical analyses
Upon examination of the data following survey completion, patterns appeared to emerge that led us to employ post hoc, one-tailed t-tests to test a posteriori hypotheses that fish species richness, family-level diversity, functional diversity, and total captures from sites inside the nature reserve were higher than from sites outside the nature reserve. Because the sampling focus shifted from fishes to freshwater decapod crustaceans during 2010 and the sampling in 2011 did not include sites outside the nature reserve, we excluded 2010 and 2011 data from these analyses. In accordance with the recommendation of Dayton [Citation80], we set alpha = 0.10 to decrease the probability of Type II error, concurring that failure to detect patterns that do exist can result in dire conservation consequences. While survey methods were consistent within each year, we elected to test each year separately due to changes in survey methods between years.
Results
Survey summaries
During the 5 years of sampling the upper Jejuí River watershed in the biosphere reserve, we collected 7,110 individuals and identified 97 fish species, belonging to seven orders, 26 families, and 66 genera (Supplemental Information). Two orders, Gymnotiformes and Cyprinidontiformes, were new records for the biosphere reserve. Of the 26 families, 14 were new records for reserve, including five within Characiformes (Acestrorhynchidae, Anostomidae, Iguanodectidae, Lebiasinidae, Parodontidae) and three within Siluriformes (Auchenipteridae, Cetopsidae, Pseudopimelodidae). The remaining six new families reside within the two newly-documented orders, Gymnotiformes (Gymnotidae, Hypopomidae, Rhamphichthyidae, Sternopygidae) and Cyprinidontiformes (Cynolebiidae, Poeciliidae). Our surveys yielded 65 species not previously identified for the biosphere reserve (Supplemental Information). Fishes were categorized into one of 14 functional groups (Supplemental Information).
Of the 48 species previously recorded for the reserve [Citation22], we detected 37 in our surveys. Of the remaining 11 species previously recorded, three have been discounted (Astyanax bimaculatus, Astyanax brevirhinus and Hemigrammus belottii) because they are known only from the Amazon River basin [Citation72]. The remaining eight species were not detected in our surveys and are so noted in the Supplemental Information. When added to the 97 species detected by our surveys, a minimum fish species richness of 105 is obtained for the biosphere reserve. Characiformes represented 57.1% of the species (60) while Siluriformes represented 31.4% (33).
Of the 97 species collected overall, 25 were captured during each of the 5 years and may thus be considered common (Supplemental Information). Common species represented 10 families. Only three of 25 (12.0%) were from orders other than Characiformes or Siluriformes: one each gymnotiform (Brachyhypopomus bombilla), synbranchiform (Synbranchus marmoratus), and cichliform (Crenicichla lepidota). In contrast, 29 species were detected only during a single year; we consider them rare. Rare species belonged to 13 families. Poeciliidae (Phallotorynus victoriae) and Belonidae (Potamorrhapis eigenmanni) were represented exclusively by rare species (Supplemental Information). Comparatively more species of rare fishes were from orders other than Characiformes or Siluriformes (8 of 28, 28.6%): four gymnotiforms (Bachyhypopomus draco, Gymnotus carapo, G. pantanal, G. paraguensis), two cyprinodontiforms (Melanorivulus cyanopterus, Phallotorynus victoriae), and one each cichliform (Apistogramma borellii) and beloniform (Potamorrhapis eigenmanni).
From our 2007 collections, we identified 62 species, 33 of which represented new records for the biosphere reserve. We identified 53 species in 2008 collections, eight of which were new for the reserve. Our most productive sampling year was 2009, yielding 71 species, of which 13 were new for records for the reserve. Total species identified and species new to our collections both declined during 2010 and 2011. In 2010, we identified 47 species of which six were new records for the reserve, and in 2011, we identified 44 species, of which two were new biosphere reserve records.
Invertivores were the most common diet type (44.3% of species) followed by omnivores (24.7%), algivores (13.4%), detritivores (11.3%), and piscivores (6.2%). Benthic fishes were the most common foraging stratum (36.1%) followed closely by benthopelagic fishes (35.1%). Mesopelagic were less common (21.6%) while surface fishes were comparatively uncommon (7.2%). The most common functional group was benthopelagic invertivores (15.5% of species) followed by mesopelagic invertivores (12.4%), benthic algivores (12.4%), and benthic invertivores (11.3%). The least common functional groups were mesopelagic algivores and benthic piscivores (both 1.0%).
Richness and diversity
Species richness, family-level diversity, and functional diversity were higher inside the nature reserve than outside the nature reserve (but still within the biosphere reserve) for the three years tested, while no significant differences occurred in the total number of individuals caught inside compared to outside during each survey year ().
Table 2. Mean ± standard deviation for species richness, family-level diversity, functional diversity, and total number of fish collected at sites inside Mbaracayú Forest Nature Reserve compared to sites outside Mbaracayú Forest Nature Reserve from survey years 2007, 2008, and 2009
In 2007, the number of fish species at sites within the nature reserve varied from 3 to 23 while the number outside the nature reserve ranged from 2 to 14, and the number of individuals captured varied from 22 to 240 inside to 9 to 166 outside the nature reserve. Richness inside the reserve was 82.3% greater than that found outside the reserve (one-tailed t = 2.14, df = 15, p = 0.028; )). Family-level diversity inside the reserve was 40.6% higher than outside the reserve (one-tailed t = 1.82, df = 15, p = 0.047; )) while functional diversity was 60.9% greater inside the reserve compared to outside (one-tailed t = 3.51, df = 15, p = 0.002; )). While not statistically significant, a greater number of individuals was collected from inside the reserve than outside in 2007 (one-tailed t = 1.36, df = 15, p = 0.168; )).
Figure 3. Comparisons of diversity measures for fishes captured inside vs. outside the Mbaracayú Forest Nature Reserve for survey years 2007, 2008, and 2009: (A) mean fish species richness (S) ± standard deviation, (B) mean family-level diversity ± standard deviation, (C) mean functional diversity ± standard deviation, and (D) mean number of individual fish collected ± standard deviation.
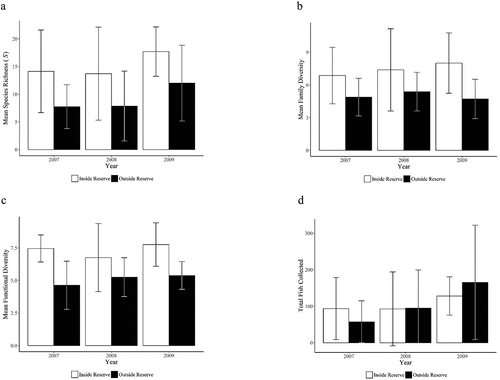
In 2008, the number of fish species at sites inside nature reserve varied from 3 to 25 while the number outside the nature reserve ranges from 3 to 21, and the number of individuals captured varied from 17 to 319 inside and 7 to 324 outside the nature reserve. Richness inside the nature reserve was 74.4% greater than outside the reserve (one-tailed t = 1.36, df = 15, p = 0.084; )). Family-level diversity was 37.2% higher inside the nature reserve than outside (one-tailed t = 1.486, df = 15, p = 0.083; )) while functional diversity was 28.6% greater inside the reserve than outside (one-tailed t = 1.41, df = 15, p = 0.093; )). In 2008, more individuals were collected from outside the reserve, though no significant difference was detected (one-tailed t = 1.34, df = 15, p = 0.478; )).
In 2009, the number of fish species at sites inside the nature reserve varied from 11 to 23 while the number outside the nature reserve ranged from 4 to 23, and the number of individuals captured varied from 13 to 431 outside and 50 to 184 inside the nature reserve. Richness was again significantly higher (47.5%) at sites inside the reserve compared to sites outside the reserve (one-tailed t = 1.85, df = 15, p = 0.044; )). Family-level diversity of fishes inside the reserve was 69.9% more than outside (one-tailed t = 2.63, df = 15, p = 0.012; )) while functional diversity was 44.1% higher inside the reserve than outside (one-tailed t = 3.40, df = 15, p = 0.003; )). Though not statistically significant, there were more fish collected outside the reserve than inside (one-tailed t = 0.60, df = 15, p = 0.280; ).
Discussion
The 105 species identified within the biosphere reserve represent 31.5% of the estimated 333 species for the Paraguay Freshwater Ecoregion [Citation39,Citation40]. The Paraguay Freshwater Ecoregion consists only of the immediate ecoregion associated with the left margin Paraguay River watershed and does not consider the fish species associated with the Chaco region of western Paraguay [Citation9] that are included in country-wide diversity estimates. This substantial proportion of the ecoregion’s fish fauna that occurs within the biosphere reserve highlights the importance of management in the reserve that maintains aquatic habitat quality to support healthy fish assemblages.
Our results yielded an increase in the number of fish species known to inhabit the Mbaracayú Forest Biosphere Reserve by 65 species including two new orders and 14 new families. This increase can likely be attributed to the increase in sampling effort, including sampling more of the varied aquatic habitats found there. Moreover, while results from each year’s surveys (both number of species captured and species composition) were similar to those in other studies from southern South America [Citation31–33,Citation81], the cumulative number of species detected was increased by our repeated effort over 5 years.
Twenty-five common species were detected during each and every year of the survey. Composition of this group was similar to the overall fish community composition, with Characiformes representing 52.0% (13) and Siluriformes representing 36% (9). This group of commonly encountered species in the streams of the biosphere reserve reflects the general community composition of broader fish community structure patterns throughout the neotropics [Citation39].
Not previously documented for the biosphere reserve, gymnotiforms were detected every year during our surveys. That gymnotiforms had not previously been recorded is surprising given the number of species (15) in Paraguay [Citation47], their regular occurrence in our surveys, both temporally and spatially, and their prominence in fish assemblages and widespread distributions throughout the Neotropics [Citation39,Citation82]. Detection of cyprinodontiforms did not occur until 2009, the third year of our survey, and corresponded with new survey locations, including two in Cerrado (savannah) habitat (sites 3B and 3C in and ).
The composition of fishes detected during our survey was similar to that of other fish assemblages compiled by Chernoff et al. [Citation42] for the upper Paraguay River and by Ramlow [Citation44] for all of Paraguay. While the collections reported by Ramlow [Citation44] originated from throughout Paraguay, the majority occurred in the vicinity of the Paraguay River and its immediate left margin tributaries. Therefore, it appears that the ichthyofaunal diversity of Paraguay’s Freshwater Ecoregion is well represented in the biosphere reserve, particularly in the relatively pristine forested core protected area of the nature reserve. Thus, the fish assemblage of the nature reserve may represent a model for similar headwater regions of the Paraguayan Atlantic Forest Ecoregion prior to expansive deforestation.
Significantly fewer fish species were collected at sites outside the core nature reserve, illustrating the negative impact of deforestation and land conversion on fish assemblages in these headwater streams, and demonstrating the benefit of protected areas for conservation of biodiversity. Deforestation is known to negatively impact diversity in many Neotropical aquatic systems [Citation35,Citation83]; homogenization of the physical environment around streams in the neotropics leads to reduced overall species diversity despite equal, or even greater, abundance of fish [Citation84]. We found no statistical differences in abundance of total fish captures inside the nature reserve versus outside for all three years tested, which adds support to our conclusion that results for the three diversity measures presented (species richness, family-level diversity, functional diversity) reflect real difference between years. That functional diversity was greater inside the nature reserve than outside during each year could be attributed to the decreased heterogeneity of the aquatic system with landscape changes that have occurred outside the core protected area, a situation documented in other regions of the neotropics, where deforestation negatively impacts fish communities [Citation35,Citation84–86].
Terrestrial habitat modification is a global threat to aquatic biodiversity [Citation87] and this is especially problematic for freshwater fish diversity in the Neotropical region [Citation3]. Conversion of natural habitat in Paraguay into human-altered landscapes has been extensive and has accelerated in recent decades [Citation17]. Forest cover within Paraguay’s Atlantic Forest Ecoregion was reduced from 73.4% to 24.9% between the 1970ʹs and 2000ʹs, and this reduction occurred throughout the region irrespective of proximity to protected areas [Citation13,Citation17]. This rapid loss in forest cover is causing major declines in biodiversity within Paraguay, highlighting the importance of conserving natural areas [Citation88]. Considering that the fish fauna of the Mbaracayú Forest Biosphere Reserve includes approximately one-third of the regional fish diversity, management of the reserve is extremely important for maintaining what is likely an excellent representation of fish assemblages in headwaters of the Paraguay Freshwater Ecoregion. As landscape change continues due to increasing agricultural and urbanization demands, the value of the biosphere reserve for conserving fish diversity is likely to increase. Managers of the biosphere reserve should consider the impact of actions not only on the terrestrial environment but also on the aquatic environment.
Author contributions
GLZ, LJ, JCM, FRP, and MV initiated this project. All co-authors participated in fish surveys. GLZ, LJ, DRE, JCM, AB, and FRP identified specimens in the field. GLZ and LJ identified specimens in the lab. FR-P, SF, and MV managed logistical support at the Mbaracayú Forest Biosphere Reserve. GLZ and DRE wrote the manuscript. DEK managed the GIS database and developed maps. All co-authors reviewed and approved the manuscript.
Supplemental Material
Download MS Word (33.6 KB)Acknowledgments
We thank the many University of Dubuque students who assisted with fish surveys. Elizabeth Bainbridge, Gretchen Breitbach, WynLyn (Brunson) McBride, Jonathan Colyer, Elizabeth Dawn, Elizabeth Dunn, Frances (Eggers) Owen, Bridgette Fidder, Mikaela (Tully) Foust, Christine Grannis, Megan (Johnson) Sprague, Kayleen (Keehner) Griep, Mackenzie Kissell, Christopher Kuhle, Arthur Magee, Kevin Mathias, Aaron Matthews, Andrew McDonnell, Matthew O’Brien, Jake O’Rourke, Amy (Stutzman) Satterlee, Benjamin Swan, Nicole Tscharner, and all participated in fish surveys as part of a summer class experience. We also thank the FMB rangers who oversaw the field work at the Mbaracayú Forest Biosphere Reserve: Osvaldo Carrillo, Osvaldo Fernandez, and Silverio Ramirez. Hector Vera Alcaraz provided advice for field work and fish identifications. We are grateful to the National Mississippi River Museum and Aquarium, the University of Dubuque, and Emporia State University’s Faculty Research and Creativity Committee for funding.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Albert JS, Tagliacollo VA, Dagosta F. Diversification of Neotropical freshwater fishes. Annu Rev Ecol Evol S. 2020;51(1):27–53.
- Barton JR. Chapter 16. Environment and natural resources. In: Heenan P, Lamontagne M, editors. The South American handbook. London (UK): Routledge; 2013. p. 179–189.
- Pelicice FM, Bialetzki A, Camelier P, et al. Human impacts and the loss of neotropical freshwater fish diversity. Neotrop Ichthyol. 2021;19(3):1–15.
- Rodríguez-Olarte D, Taphorn DC, Lobón-Cerviá J. Do protected areas conserve neotropical freshwater fishes? A case study of a biogeographic province in Venezuela. Anim Biodiv Conserv. 2011;34(2):273–285.
- Oliveira AG, Peláez O, Agostinho AA. The effectiveness of protected areas in the Parana-Paraguay basin in preserving multiple facets of freshwater fish diversity under climate change. Neotrop Ichthyol. 2021;19(3):e210034.
- Skelton GA, Vambray PH, Lombard JA, et al. Patterns of distribution and conservation status of freshwater fishes in South Africa. Afr Zool. 1995;20:71–81.
- Rodrigues ASL, Akçakaya HR, Andelman SJ, et al. Global gap analysis: priority regions for expanding the global protected-area network. BioScience. 2004;54:1092–1100.
- Di Bitetti MS, Placci G, Dietz LA, et al. A biodiversity vision for the Upper Paraná Atlantic Forest Ecoregion: designing a biodiversity conservation landscape and setting priorities for conservation action. Washington (DC): World Wildlife Fund; 2003.
- Olson DM, Dinerstein E, Canevari P, et al. editors. Freshwater biodiversity of Latin America and the Caribbean: a conservation assessment. Washington (DC): World Wildlife Fund Biodiversity Support Program; 1998.
- Abell R, Thieme ML, Revenga C, et al. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. BioScience. 2008;58(5):403–414.
- Fleytas C. Cambios en el paisaje. Evolución de la cobertura vegetal en la región Oriental del Paraguay. In: Salas-Dueñas D, Facetti J, editors. Biodiversidad del Paraguay: una imacion a Sus Realidades. 1st ed. Asuncion (Paraguay): Fundación Moises Bertoni, USAID, GEF/BM; 2007. p. 77–88.
- Hansen MC, DeFries RS. Detecting long-term global forest change using continuous fields of tree-cover maps from 8-km advanced very high resolution radiometer (AVHRR) data for the years 1982-99. Ecosystems. 2004;7(7):695–716.
- Huang C, Kim S, Alstatt A, et al. Rapid loss of Paraguay’s Atlantic forest and the status of protected areas – a Landsat assessment. Remote Sens Environ. 2007;106(4):460–466.
- Da Ponte E, Mack B, Wohlfart C, et al. Assessing forest cover dynamics and forest perception in the Atlantic forest of Paraguay, combining remote sensing and household level data. Forests. 2017;8(10):1–21.
- Hansen MC, Stehman SV SV, Potapov PV. Quantification of global gross forest cover loss. Proc Natl Acad Sci U S A. 2010;107(19):8650–8655.
- Hansen MC, Potapov PV, Moore R, et al. High-resolution global maps of 21st-century forest cover change. Science. 2013;342(6160):850–853.
- Huang C, Kim S, Song K, et al. Assessment of Paraguay’s forest cover change using Landsat observations. Global Planet Change. 2009;67(1–2):1–12.
- Lovera M The environmental and social impacts of unsustainable livestock farming and soybean production in Paraguay. XIV World Forestry Congress. Durban: South Africa, Sept. 2015. http://foris.fao.org/wfc2015/api/file/55e1845cb283bd1b116fbb8a/contents/1e94cdc6-7fe5-4428-a5e4-4264d4ec9775.pdf
- Richards PD. Soy, cotton, and the final Atlantic frontier. Prof Geogr. 2011;63(3):343–363.
- Iwata T, Nakano S, Inoue M. Impacts of past riparian deforestation on stream communities in a tropical rain forest in Borneo. Ecol Appl. 2003;13(2):461–473.
- Luke SH, Barclay H, Bidin K, et al. The effects of catchment and riparian forest quality on stream environmental conditions across a tropical rainforest and oil palm landscape in Malaysian Borneo. Ecohydrology. 2017;10(4):1–14.
- Fundación Moisés Bertoni/Banco Mundial. Reserva Natural del Bosque Mbaracayú. Plan de manejo 2005-2010. Asunción: Paraguay: Fundación Moisés Bertoni para la Conservación de la Naturaleza (FMB); 2005.
- Cacciali P. Diversidad de anfibios y reptiles en Paraguay. In: Salas-Dueñas DA, Facetti JF, editors. Biodiversidad del Paraguay, una aproximación a sus realidades. Asunción (Paraguay): Fundación Moisés Bertoni, USAID, GEF/BM; 2007. p. 109–118.
- Fragano F, Clay R. Biodiversity status of the interior Atlantic Forest of Paraguay. In: Gallindo-Leal C, de Gusmão Câmara I, editors. The Atlantic Forest of South America: biodiversity status, threats, and outlook. Washington (DC): Island Press; 2003. p. 288–309.
- Morales M. Diversidad de mamíferos en Paraguay. In: Salas-Dueñas DA, Facetti JF, editors. Biodiversidad del Paraguay, una aproximación a sus realidades. Asunción (Paraguay): Fundación Moisés Bertoni, USAID, GEF/BM; 2007. p. 133–150.
- Velazquez M. Diversidad de aves en Paraguay. In: Salas-Dueñas DA, Facetti JF, editors. Biodiversidad del Paraguay, una aproximación a sus realidades. Asunción (Paraguay): Fundación Moisés Bertoni, USAID, GEF/BM; 2007. p. 119–132.
- Valério SB, Súarez YR, Felipe TRA, et al. Organization patterns of headwater-stream fish communities in the Upper Paraguay-Paraná basins. Hydrobiologia. 2007;583(1):241–250.
- Albert JS, Reis RE. Introduction to neotropical freshwaters. In: Albert JS, Reis RE, editors. Historical biogeography of neotropical freshwater fishes. Berkeley: University of California Press; 2011. p. 3–19.
- Oliveira EF, Minte-Vera CV, Goulart E. Structure of fish assemblages along spatial gradients in a deep subtropical reservoir (Itaipu Reservoir, Brazil-Paraguay border). Environ Biol Fish. 2005;72(3):283–304.
- Oliveira VA, Mateus LA, Loverde-Oliveira S, et al. Fish from urban tributaries to the Vermelho River, upper Paraguay River Basin, Mato Grosso, Brazil. Check List. 2015;11(1):1–6.
- Menni RC, Miquelarena AM, Lopez HL, et al. Fish fauna and environments of the Pilcomayo-Paraguay basins in Formosa, Argentina. Hydrobiologia. 1992;245(3):129–146.
- Teresa FB, Romero R, Langeani FP. Aquidauana and Miranda drainages, upper Paraguay River basin, Mato Grosso do Sul, Brazil. Check List. 2010;6(4):596–601.
- Flores SA, Araya PR, Serrano MJ, et al. Estructura de la comunidad íctica del arroyo Paraíso afluente del río Uruguay. Misiones Argentina Biol Acuat. 2020;34:1–12.
- Lowe-McConnell RH. . In: Fish communities in tropical freshwaters: their distribution, ecology and evolution. New York: Longman Publishing Group; 1975. 337 pp.
- Bojsen BH, Barriga R. Effects of deforestation on fish community structure in Ecuadorian Amazon streams. Freshwater Biol. 2002;47(11):2246–2260.
- Gorman OT, Karr JR. Habitat structure and stream fish communities. Ecology. 1978;59(3):507–515.
- Guégan J-F, Lek S, Oberdorff T. Energy availability and habitat heterogeneity predict global riverine fish diversity. Nature. 1998;391(6665):382–384.
- Winemiller KO, Flecker AS, Hoeinghaus DJ. Patch dynamics and environmental heterogeneity in lotic ecosystems. J North Am Benthol Soc. 2010;29(1):84–99.
- Albert JS, Petry P, Reis RE. Major biogeographic and phylogenetic patterns. In: Albert JS, Reis RE, editors. Historical biogeography of neotropical freshwater fishes. Berkeley: University of California Press; 2011. p. 21–57.
- Carvalho TP, Albert JA. The Amazon-Paraguay divide. In: Albert JS, Reis RE, editors. Historical biogeography of neotropical freshwater fishes. Berkeley: University of California Press; 2011. p. 193–202.
- Swedish Museum of Natural History Ichthyology Database [Internet]. Stockholm (Sweden): Swedish Museum of Natural History; 2020 cited 2020 Feb 18]. Available from 2020 Feb 18: http://artedi.nrm.se/nrmfish/find.php?FormData=PARAGUAY&Category=country&Precision==&MaxRecs=10000&Extent=All&Verbosity=Listing&Fulltext=No&Map=Map&Ordering=scientificName&Direction=ASC
- Chernoff B, Willink PW, Montambault JR, editors A biological assessment of the río Paraguay basin, Alto Paraguay, Paraguay. In: RAP Bulletin of Biological Assessment. Vol. 19. Washington (DC): Conservation International; 2001. 156 pp.
- Vera Alcaraz HS, Del Castillo H. Capitulo 4. Peces del río Paraguay. In: Morales C, Yanosky A, Luna L editors. Biodiversidad del río Paraguay. Asunción. (Paraguay): Asociación Guyra Paraguay Transbarge Navegación; 2006. p. 40–58.
- Ramlow JM. Lista de peces y sitios de colección de la Sección de Ictiología del Inventario Biológico Nacional/Museo Nacional de Historia Natural del Paraguay (Junio, 1980 – diciembre, 1988). Bol Inv Biol Nac Paraguay. 1989;9:1–40.
- FishBase [Internet]. Kiel (Germany): MySQL; 2020 [cited 2020 Feb 18]. “All fishes reported from Paraguay (landlocked)”. Available from: http://www.fishbase.org/.
- FAUNA Paraguay [Internet]. Encarnación (Paraguay): FAUNA Paraguay; 2020 [2020 Jan 20]. “List of the Fish of Paraguay”. Available from: http://www.faunaparaguay.com/fishlist.html.
- Koerber S, Vera-Alcaraz HS, Reis RE. Checklist of the fishes of Paraguay (CLOFPY). Ichthyol Contr Peces Criollos. 2017;53:1–99.
- Satterlee SA, Zuercher GL, Kuhle CW, et al. Rediscovery of aeglid crabs in the rio Jejuí watershed, Paraguay. J Crustacean Biol. 2012;32(4):541–543.
- Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Zool Univ Mich. 2001;190:1–127.
- Albert JS, Crampton WGR. Diversity and phylogeny of neotropical electric fishes (Gymnotiformes). In: Bullock TH, Hopkins CD, Popper AN editors. Electroreception. New York: (NY): Springer; 2005. p. 360–409.
- Britski HA, Silimon KZS, Lopes BS. Peixes do Pantanal: manual de identificação. Brasília (Brazil): Embrapa, Serviço de Produção de Informação; 1999.
- Casciotta J, Almirón A, Bechara J. Peces del Iberá: hábitat y diversidad. La Plata. (Argentina): Grafikar; 2005.
- Collette B. South American freshwater needlefishes of the genus Potamorrhaphis (Beloniformes: belonidae). P Biol Soc Wash. 1982;95:714–747.
- Costa WJEM. Seven new species of the killifish genus Rivulus (Cyprinodontiformes: rivulidae) from the Paraná, Paraguay and upperAraguaia river basins, central Brazil. Neotrop Ichthyol. 2005;3(1):1–19.
- Covain R, Fisch-Muller S. The genera of neotropical armored catfish subfamily Loricariinae (Siluiformes: loricariidae): a practical key and synopsis. Zootaxa. 2007;1462(1):1–40.
- Géry J, Mahnert V, Dlouhy C. Poissons Characoïdes non Characidae du Paraguay (Pisces, Ostariophsyi). Rev Suisse Zool. 1987;94:357–464.
- Koch WR. Revisão taxonômica do gênero Homodiaetus (Teleostei, Siluriformes, Trichomycteridae).Iheringia. Ser Zool. 2002;92:33–46.
- Kullander SO. A revision of the South American cichlid genus Cichlasoma (Teleostei: cichlidae). Stockholm (Sweden): The Swedish Museum of Natural History; 1983.
- Lucinda PHF, Rosa RS, Reis RE. Systematics and Biogeography of the Genus Phallotorynus Henn, 1916 (Cyprinodontiformes: poeciliidae: poeciliinae), with Description of Three New Species. Copeia. 2005;2005(August):609–631.
- Menezes NA, Weitzman SH. Two new species of Mimagoniates (Teleostei: characiformes: characidae), their phylogeny and biogeography and a key to glandulocaudin fishes of Brazil and Paraguay. P Biol Soc Wash. 1990;103:380–426.
- Mirande JM. Phylogeny of the family Characidae (Teleostei: characiformes): from characters to taxonomy. Neotrop Ichthyol. 2010;8(3):385–568.
- Neris N, Köhn C, Villalba F, et al. Guía ilustrada de los peces más comunes del Paraguay. Asunción (Paraguay): Artes Gráficas Zamphiropolos SA; 2008.
- Neris N, Villalba F, Kamada D, et al. Guía de peces del Paraguay. Asunción (Paraguay): Artes Gráficas Zamphiropolos SA; 2010.
- Sarmento-Soares LM, Martins-Pinheiro RF. A systematic revision of Tatia (Siluriformes: auchenipteridae: centromochlinae). Neotrop Ichthyol. 2008;6(3):495–542.
- Schindler I, Etzel V. Re-description and distribution of Rivulus punctatus Boulenger, 1895 (Teleostei: rivulidae) and its habitats in Paraguay. Vertebr Zool. 2008;58:33–43.
- Vari RP. Notes on the characoid subfamily Iguanodectinae, with a description of a new species. Am Mus Novit. 1977;2612:1–6.
- Vari RP. Systematics of the neotropical characiform genus Steindachnerina Fowler (Pisces: ostariophysi). Sm C Zool. 1991;507:1–118.
- Vari RP. Systematics of the Neotropical characiform genus Curimatella Eigenmann and Eigenmann (Pisces: ostariophysi) with summary comments on the Curimatidae. Sm C Zool. 1992;533:1–48.
- Vari RP, Harold AT. Phylogenetic study of the neotropical fish genera Creagrutus Günther and Piabina Reinhardt (Teleostei: ostariophysi: characiformes), with a revision of the Cis-Andean species. Sm C Zool. 2001;613:1–239.
- Vera Alcaraz HS, da Graça WJ, Shibatta OA. Microglanis carlae, a new species of bumblebee catfish (Siluriformes: pseudopimelodidae) from the río Paraguay basin in Paraguay. Neotrop Ichthyol. 2008;6(3):425–432.
- Vera Alcaraz HS, Pavanelli CS, Zawadzki CH. Taxonomic revision of the Rineloricaria species (Siluriformes: loricariidae) from the Paraguay River basin. Neotrop Ichthyol. 2012;10(2):285–311.
- Fricke R, Eschmeyer WN, Van der Laan R, editors. Eschmeyer’s catalog of fishes: genera, species, references. Electronic version. San Francisco (CA): California Academy of Sciences; 2021. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
- Ibañez C, Tedesco P, Bigorne R, et al. Dietary-morphological relationships in fish assemblages of small forested streams in the Bolivian Amazon. Aquat Living Resour. 2007;20(2):131–142.
- Winemiller KO, Agostinho AA, Caramaschi EP. Fish ecology in tropical streams. In: Dudgeon D, editor. Tropical stream ecology. London (UK): Academic Press; 2008. p. 107–146.
- Casatti L, Teresa FB, Zeni JO, et al. More of the same: high functional redundancy in stream fish assemblages from tropical agroecosystems. Environ Manage. 2015;21:433–442.
- Pease AA, González-Díaz AA, Rodiles-Hernández R, et al. Functional diversity and trait-environment relationships of stream fish assemblages in a large tropical catchment. Freshw Biol. 2012;57(5):1060–1075.
- Souza CP, Rodrigues-Filho CAS, Barbosa FAR, et al. Drastic reduction of the functional diversity of native ichthyofaunal in a Neotropical lake following invasion by piscivorous fishes. Neotrop Ichthyol. 2021;19(3):e210033.
- Toussaint A, Charpin N, Brosse S, et al. Global functional diversity of freshwater fish in concentrated in the Neotropics while functional vulnerability is widespread. Sci Rep. 2016;6(1):22125.
- Root RB. The niche exploitation pattern of the blue-gray gnatcatcher. Ecol Monogr. 1967;37(4):317–350.
- Dayton PK. Reversal of the burden of proof in fisheries management. Science. 1998;279(5352):821–822.
- Pavanelli CS, Caramaschi ÉP. Temporal and spatial distribution of the ichthyofauna in two streams of the upper rio Paraná basin. Braz Arch Biol Techn. 2003;46(2):271–280.
- Crampton. WGR An ecological perspective on diversity and distributions. In: Albert JS, Reis RE, editors. Historical biogeography of neotropical freshwater fishes. Berkeley: University of California Press; 2011. p. 165–189.
- Teresa FB, Casatti L. Influence of forest cover and mesohabitat types on functional and taxonomic diversity of fish communities in neotropical lowland streams. Ecol Freshw Fish. 2012;21(3):433–442.
- Zeni JO, Casatti L. The influence of habitat homogenization on the trophic structure of fish fauna in tropical streams. Hydrobiologia. 2014;726(1):259–270.
- Brook BW, Sodhi NS, Ng PKL. Catastrophic extinctions follow deforestation in Singapore. Nature. 2003;424(6947):420–426.
- Toham AK, Teugels GG. First data on an Index of Biotic Integrity (IBI) based on fish assemblages for the assessment of the impact of deforestation in a tropical West African river system. Hydrobiologia. 1999;397:2–38.
- Allan JD, Flecker AS. Biodiversity conservation in running waters. BioScience. 2003;43(1):32–43.
- Yanosky A. Paraguay’s challenge of conserving natural habitats and biodiversity with global markets demanding for products. In: Sodhi NS, Gibson L, Raven PH, editors. Conservation biology: voices from the tropics. West Sussex (UK): John Wiley & Sons, Ltd.; 2013. p. 113–119.
