ABSTRACT
Tlaloc hildebrandi is a small (<130 mm) freshwater fish endemic to Southwest Mexico and is listed as endangered through habitat loss and invasive species. We determine and compare the parameters of the length–weight relationship, relative weight, and condition factor among different populations of the endemic killifish, T. hildebrandi. Fulton’s condition factor (K), relative weight (Wr), and the length–weight relationship were estimated for three killifish populations throughout its distribution range in the highlands of Chiapas, México. The Wr was significantly lowest in fishes where there are higher anthropogenic activities. The somatic indexes and the length–weight relationships for T. hildebrandi are hereby published for the first time in both the scientific literature and databases, such as Fishbase.
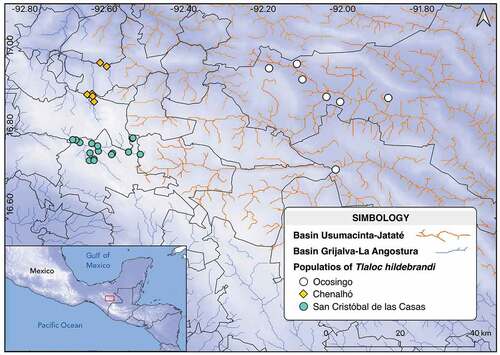
Tlaloc hildebrandi (Miller, 1950), known as Chiapas killifish, is a freshwater fish endemic to highlands of Chiapas, Mexico. This species is listed as threatened by IUCN and Mexican law in the endangered category [Citation1,Citation2] due to its low abundance and confined distributional range [Citation3]. Recently, this species has been subject to environmental pressures due to habitat alterations in the Grijalva River basin produced by urban growth in the valley of San Cristóbal de Las Casas, Chiapas. Nonetheless, new populations have been recently discovered in the upper reaches of the high Usumacinta River in Chiapas, México [Citation4].
Corporal indices have been used widely for fisheries management, conservation, and biomonitoring of environmental stress on fish health. Most commonly used are somatic indexes such as condition factor (CF), relative weight (RW), and length–weight relationship (LWR) [Citation5,Citation6]. Thus, this study aimed to determine and compare the parameters of the length–weight relationship, relative weight, and condition factor among different populations of the endemic killifish, T. hildebrandi, through its distribution range, in order to enhance the conservation of this genetic resource.
Fish samples were collected in 18 localities of three populations [Citation7], two populations belonging to the Grijalva basin (The Valley of San Cristóbal and The Chenalhó River basin), and one from the Usumacinta River (Ocosingo) [], from December 2014 to August 2017. In the field, specimens were fixed in a 10% formaldehyde solution, stored for 2 days, and then transferred to a 70% ethanol solution for final preservation. The standard length (mm) and weight (g) measurements were taken from all the specimens of each population.
The fish length–weight relationships were considered to be allometric growth models. The LWR was determined for each population and all specimens using the allometric equation [Citation8]. Relative weight was calculated for each fish as Neumann et al. [Citation9]. Fulton’s condition factor (K) was calculated using the equation of Fulton [Citation10].
Since our data did not follow the assumptions for parametric measurement, a Kruskal-Wallis was implemented in order to test for significant differences between the different populations for both somatic indexes: Wr and K. The statistical significance level of the coefficient of determination (r2) and 95% confidence intervals (95%CI) of a and b in the LWR relationships were also estimated [Citation11]. All analyses were performed with the use of the statistical software R 3.0.2 [Citation12] using the “CAR” package [Citation13].
A total of 632 fish individuals were collected (scientific collection permit: SGPA/DGVS/00488/16). The population name, sample size (n), minimum and maximum standard length, intercept a, slope b, 95% confidence intervals of a and b, and coefficient of determination (r2) are summarized in . The LWR relationship for the Chiapas killifish along assessed populations, showed a positive allometric growth (b = 3.156) (LLb = 3.134, ULb = 3.178). However, the separate analysis of the three populations showed significant variations in the value of b; the parameter b ranged from 2.904 (isometric growth) (Ocosingo) to 3.198 and 3.193 (positive allometric growth) (Chenalhó and San Cristóbal, respectively). All relations were statistically significant (P < 0.001), with high r2 values ranging from 0.945 (Ocosingo) to 0.996 (Chenalhó) [].
Table 1. Parameters of the length–weight relationship of T. hildebrandi in three populations the highlands of Chiapas, México.
The average relative weight (Wr) for all combined populations was 1.00 (SD = 0.19); with the San Cristóbal population having the lowest average value (0.96, SD = 0.13), while the highest average value was found in the Ocosingo population (1.02, SD = 0.32) []. Significant differences were found in the relative weight between populations (H = 22.695, P < 0.0001).
Table 2. Values of the relative weight (Wr) and the index of condition Fulton (K) of T. hildebrandi in three populations of different municipalities of the highlands of Chiapas, México.
Futon’s condition index (K) for all combined populations has an average value of 1.88 ± 0.36, with the lowest average value for the Ocosingo population (1.82 ± 0.52) and the highest average value for the San Cristóbal population (1.94 ± 0.22) []. Significant differences were found among the compared populations in the condition factor K (H = 45.065, p < 0.0001).
Condition indices provide a useful assessment of the plumpness and physiological well-being of fishes. Such indices provide an indirect means of evaluating ecological relations and the effects of various management strategies [Citation14]. In addition, the corporal indices have been commonly used for biomonitoring of environmental stress on fish health [Citation15–17]. In this study, Wr was significantly lowest in fishes from San Cristóbal where there are higher anthropogenic activities [Citation3]. These Wr values cannot be related to fish sex or reproductive processes because the fish samples were collected in different periods. Data collected throughout the sampling period are not representative of a particular season. Therefore, the estimated parameters Wr should be treated as mean annual values. Although our study has not investigated the influence of environmental stress on T. hildebrandi, the lowest values of Wr in fish from San Cristóbal could be related to a higher presence of environmental stressors (sewage water, parasites, and invasive species, among others) [Citation3]. The introduction of invasive alien species has been documented in San Cristobal (Micropterus salmoides, Oncorhynchys mykiss, Cyprinus carpio); further risk factors related to urban growth included sewage pollution of streams, as well as habitat destruction (lakes dried up for house construction or maize culture), fragmentation (dams), and modification (destruction of riparian vegetation, garbage accumulation, silting) [Citation3]. Despite the above, there is still little literature related to the physical-chemical parameters of water for the other two sites (Chenalhó and Ocosingo). However, some parameters documented in the Chenalhó River indicate a better quality of water at this site [Citation18].
The spatial variation in the type of growth (b), as well as the significant difference in the value of weight and condition factor of the Chiapas killifish (T. hildebrandi), could be influenced by multiple factors both biotic and abiotic related to the temperature, sex, season, stage of maturity, stomach content, competition, food availability, habitat quality and human perturbation among other factors [Citation19,Citation20].
The values of a and b for the three populations in this study were within the limits reported by Froese [Citation5], although these parameters varied significantly among the populations studied. We recorded that the exponent b values were higher than 3 (b > 3) in two populations (Chenalhó and San Cristóbal); thus, both populations could be categorized as populations displaying an allometric positive growth pattern, while the population of Ocosingo exhibited an isometric growth pattern (b = 3). LeCren [Citation21] pointed out that intraspecific variations in the length–weight relationship might be because of variations in ecological conditions of habitats and physiology or either of them, which were not considered in the present study.
Finally, our study provides an estimate of somatic indices and length–weight relationships for T. hildebrandi, and these results are published for the first time in both the scientific literature and databases, such as Fishbase [Citation22]. This information has very interesting implications for the conservation efforts in the freshwater fish diversity in regions of scarce information with high conservation priority, such as the Chiapas highlands basins. Endemic taxa may become extinct before population-level studies are completed, but such studies should form part of the basis for any conservation program. Due to the low genetic diversity of T. hildebrandi [Citation7], it is necessary to establish ex situ reproductive stocks as a first step in the conservation of its genetic diversity. This, along with in situ strategies, will allow for the persistence of the species. Particular conservation efforts should be directed to the population of San Cristóbal (Amarillo subbasin) due to strong environmental pressure, low catches per unit of effort (a small population size), and low genetic flow with the rest of the populations.
Acknowledgments
We thank the staff of the UNICACH museum of zoology, especially to Adán E. Gómez González+, Jorge Lievano, Jesús Hernández and Alejandro Jamangapé, for the support in fieldwork. We thank Diego Ardon and Alejandro Nettel for their contribution in the revision of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Diario Oficial (DOF). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección Ambiental–Especies nativas de México de flora y fauna silvestres–Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio–lista de especies en riesgo. Diario Oficial de la Federación. 30 de diciembre de. Segunda Sección: México: D.F. 2010, 78.
- Schmitter-Soto J, Vega-Cendejas M. Tlaloc hildebrandi. The IUCN red list of threatened species 2019: e.T169366A1274187 [ Downloaded on 21 January 2020]. Available from: https://dx.doi.org/10.2305/IUCN.UK.2019-2.RLTS.T169366A1274187.en.
- Velázquez-Velázquez E, Schmitter-Soto JJ. Conservation status of the San Christóbalpupfish Profundulus hildebrandi miller(Teleostei: profundulidae) in the face of urban growth in Chiapas. Mexico. Aquatic Conservation: Marine and Freshwater Ecosystems. 2004;14(2):201–209.
- Domínguez-Cisneros SE, Velázquez-Velázquez E, Anzueto-Calvo M. Ampliación de la distribución geográfica del popoyote de San Cristóbal Tlaloc hildebrandi (Miller 1950), (Cyprinodontiformes: profundulidae). LACANDONIA. 2017;11(2):13–18.
- Froese R. Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J Appl Ichthyol. 2006;22(4):241–253.
- Montenegro D, Gonzalez MT. Evaluation of somatic indexes, hematology and liverhistopathology of the fish Labrisomus philippii from San Jorge Bay, northern Chile, as associated with environmental stress. Rev Biol Mar Oceanogr. 2012;47(1):99–107.
- Beltrán-López RG, González-Díaz AA, Soria-Barreto M, et al. Genetic diversity and structure of one of the most endangered freshwater fish species in Mexico: Tlaloc hildebrandi (Miller, 1950) and recognition of its evolutionarily significant units. PeerJ. 2021;9:e11952.
- Ricker WE. Linear regressions in fisheries research. J Fish Res Board Can. 1973;30(3):409–434.
- Neumann RM, Guy CS, Willis DW. Length, weight, and associated indices. In: Fisheries techniques. 3rd ed. Bethesda: Maryland: American Fisheries Society; 2012. p. 637–676.
- Fulton TW. The rate of growth of fishes. Twenty-second Annual Report, 1904;141–241.
- Zar JH. Análisis bioestadístico. 4th ed. Nueva Jersey (NJ): Prentice Hall Press; 1999.
- R Development Core Team. Version 2013 (accessed 2017 Jan 26). Available from. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. http://www.R-project.org
- Fox J, Weisberg S. An R companion to applied regression. 2nd ed. Thousand Oaks: CA; 2011.
- Murphy BR, Willis DW, Springer TA. The relative weight index in fisheries management: status and needs. Fisheries. 1991;16(2):30–38.
- Barker DE, Khan RA, Hooper R. Bioindicators of stress in winter flounder, Pleuronectes americanus, captured adjacent to a pulp and paper mill in St. George’s Bay, Newfoundland. Can Fish Aquat Sci. 1994;51(10):2203–2209.
- Khan RA, Payne JF. A multidisciplinary approach using several biomarkers, including a parasite, as indicators of pollution: a case history from a paper mill in Newfoundland. Parassitologia. 1997;39(3):183–188.
- Andreu-Soler A, Ruiz-Campos G. Effects of exotic fishes on the somatic condition of the endangered killifish fundulus lima (Teleostei: fundulidae) in Oases of baja California Sur, Mexico. Southw Naturalist. 2013;58(2): 192–20.
- Cruz-Maza BG. Historia de vida y ecología de Tlaloc hildebrandi (Miller, 1950) en los altos de Chiapas, México. [master’s thesis]. Tuxtla Gutiérrez (TG): Universidad de Ciencias y artes de Chiapas; 2018.
- Pauly D. Some simple methods for the assessment of tropical fish stock. FAO Fish Tech Pap. 1983;234.
- Blackwell BG, Brown ML, Willis DW. Relative weight (Wr) status and current use in fisheries assessment and management. Rev Fish Sci. 2000;8(1):1–44.
- Le Cren ED. The length–weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J Anim Ecol. 1951;20(2):201–219.
- Froese R, Pauly D (eds.). FishBase. [Version February/2017]. World Wide Web Electronic Publication. Available from: http://www.fishbase.org