ABSTRACT
It is estimated that more than 500 species of insects have been introduced to the Galápagos Islands via human activities. One of these insect invaders is the yellow paper wasp, Polistes versicolor (Olivier) (Hymenoptera: Vespidae), a social wasp native to continental South America. In Galápagos, these wasps are voracious predators of insect larvae, compete with native species for insect prey or for floral resources and are a human nuisance. Wasp suppression methods currently in use are inefficient and attract non-target species, calling for the development of species-specific attractants that can be used in baits to lure and kill wasps. To evaluate the potential for using wasp semiochemicals in baits, we determined the biochemical composition of the head, thorax, Dufour’s and venom glands of P. versicolor foragers via gas chromatography/mass spectrometry (GC/MS). Male and female wasps were tested for behavioral responses to body segment extracts from both sexes. Female body extracts consistently elicited more behavioral responses in both male and female wasps than male extracts. Females reacted to female head, thorax and abdomen (the Dufour’s and venom glands are located in the abdomen) extracts, whereas males reacted significantly to female head and thorax extracts. One male body extract, the head, elicited two significant behaviors: female wasps groomed more often, and males touched the filter paper more often compared to the blank control. Head extracts consistently changed the behavior of female and male wasps and, together with female thorax extracts, have potential as species-specific lures for yellow paper wasps. Heads were mainly composed of hydrocarbon lipids and oleamide, a ligand for odorant-binding proteins. The thorax consisted of fatty aldehydes, long-chain alkanes and fatty amide lipids. Field trials of blends of these compounds in high wasp density areas of Galápagos are the next step to confirm if any of these compounds are attractive to P. versicolor.
GRAPHICAL ABSTRACT
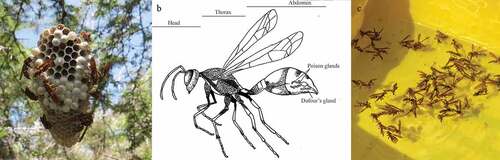
RESUMEN
Se estima que más de 500 especies de insectos han sido introducidas en las Islas Galápagos a través de actividades humanas. Uno de estos insectos invasores es la avispa de papel amarilla, Polistes versicolor (Olivier) (Hymenoptera: Vespidae), una avispa social nativa de Sudamérica continental. En Galápagos, estas avispas son predadores voraces de larvas de insectos, compiten con las especies nativas por los insectos presa o por recursos florales, además de ser una molestia para los humanos. Los métodos para controlar estas avispas que se usan en la actualidad son ineficientes y atraen a especies no objetivo, por lo cual se requiere el desarrollo de atrayentes que sean especie-específicas los cuales puedan ser usados en las trampas para cebar y matar a las avispas. Para evaluar el potencial de uso de semioquímicos de avispas en los cebos, determinamos la composición bioquímica de la cabeza, tórax, glándula de Dufour y las glándulas del veneno de forrajeras de P. versicolor usando cromatografía de gases/espectrometría de masas (GC/MS). Avispas machos y hembras se usaron en ensayos de respuestas comportamentales a extractos de segmentos del cuerpo de ambos sexos. Los extractos del cuerpo de las hembras hicieron reaccionar consistentemente más a hembras y machos que los extractos del cuerpo de machos. Las hembras reaccionaron de forma no aleatoria cuando fueron expuestas a extractos de la cabeza, el tórax y el abdomen (la glándula de Dufour y las glándulas del veneno se encuentran en el abdomen) de otras hembras. Los machos reaccionaron significativamente a extractos de la cabeza y el tórax de avispas hembra. Solo el extracto de cabeza de machos produjo una reacción significativa en dos comportamientos: las hembras realizaron aseos más frecuentemente y los machos tocaron el papel de filtro más frecuentemente en comparación con el solvente solo. Los extractos de cabeza consistentemente cambiaron el comportamiento de las avispas hembra y macho, y en conjunto con los extractos de tórax de las hembras tienen potencial como cebos especie-específicos para las avispas de papel amarillas. Las cabezas están compuestas principalmente de lípidos hidrocarbonos y oleamida, un ligando para proteínas que se unen al odorando. El tórax consiste mayoritariamente de aldehídos grasos, alcanos de cadena larga y lípidos de grasa amida. Ensayos en campo de mezclas de estos compuestos en áreas de alta densidad de avispas en las Islas Galápagos son los próximos pasos para confirmar si alguno de estos compuestos resulta atrayente para P. versicolor.
Introduction
Eusocial insects have successfully invaded many regions of the globe, causing substantial ecological damage to recipient ecosystems [Citation1,Citation2]. Several factors are identified in aiding social insects to be successful invaders; their social structure, occupation of broad niches, effective defense against predators and high dispersal capacity [Citation3]. Colony integrity and success rely heavily on the ability to distinguish nest mates from intruders. Nest mate recognition has evolved because it limits colony exploitation by parasites and individuals from other colonies. Individuals within a colony must detect specific chemical cues carrying information about the insect they are interacting with [Citation4]; thus, social insects require a more detailed chemical language than other insects [Citation5]. Among the semiochemicals used by social insects to communicate, cuticular hydrocarbons hold cues for intra- and inter-specific recognition [Citation4]. Cuticular hydrocarbons are largely non-volatile compounds that can only be perceived by an insect via direct contact such as antennation. Other odors also play a key role in kin recognition [Citation6,Citation7]. Within-colony odors vary between members and between the different phases of the colony cycle, and due to the presence of nest parasites [reviewed in 4]. In social wasps, the existence of behavioral responses to semiochemicals such as colony odors, kin recognition and thermoregulation pheromone appear to be mediated by cuticular odors rather than linked to a discrete glandular source [Citation8]. However, we still know relatively little about exocrine gland function and chemical composition in wasps.
Paper wasps in the genus Polistes have successfully invaded new habitats in several continents [Citation9–12]. Our study focuses on the yellow paper wasp, Polistes versicolor (Olivier), which is native to continental South America [Citation13]. This species was first recorded in the Galápagos archipelago in 1988 and has since invaded most of the islands [Citation14]. The ecological consequences of this invasion include predation on the islands’ insects and most likely competition with native species for food prey or for floral resources [Citation12]. In addition to its ecological impact, this wasp is a nuisance to residents and visitors alike as it affects tourism and activities in human settlements. Their stings can result in allergic reactions [Citation15]. Thus, a management plan that reduces wasp densities would benefit both wildlife and people. Our starting point for this management involves designing a targeted and attractive bait. There are currently no bait systems or known attractive substances exclusive for paper wasps [Citation16]. Some attractants associated with fermented food are used in lures with varying degrees of efficacy [Citation17]. Control methods currently available range from relatively benign solutions, such as pan traps filled with soapy water, to generic insecticide sprays, through to the more drastic burning of colonies [Citation18].
Semiochemicals produced in the body or glands of Polistes wasps have potential as attractants for use in trapping programs yet are relatively unexplored. Cuticular hydrocarbons of Polistes wasps are composed of species-specific mixtures of compounds [Citation4]. Chemical analyses showed that a nest might carry the same odors of the wasps occupying the combs, and behavioral assays determined that the compounds covering the nest surface are necessary for the correct learning of nestmate recognition cues [Citation4]. Studies on different Polistes species indicated that the secretions of some of the head glands may be pheromones distributed by trophallaxis between adults, fed to larvae or added to the nest materials [Citation19]. In different species of Polistes, different glands play a role in communication. For example, in P. exclamans, the thorax seems to be the source of female pheromones, while the legs, gastral sternal glands and mandibular glands are thought to be the sources of a male pheromone [Citation20].
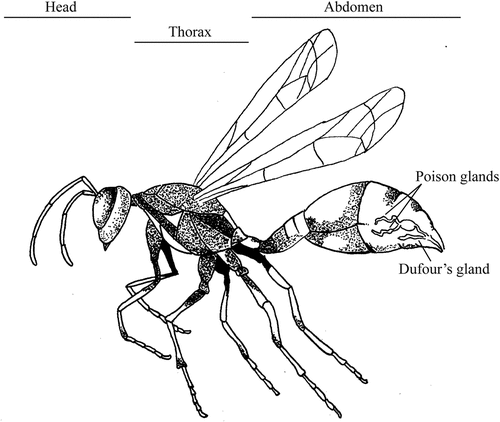
In eusocial wasps, glands with potential to produce pheromones are found in all parts of the body. Eight pairs of glands have been identified in female heads, all associated with the mouthparts or oral cavity [Citation19,Citation21]. The mouthparts are used for ingestion, killing prey, paper making, soil excavation, trophallaxis, grooming, nest cleaning and mauling [Citation22]. There is only one gland known from the wasp thorax, the thoracic gland, also known as the labial or salivary gland [Citation19]. In adult female paper wasps, the thoracic gland produces the cement which helps to glue the wood pulp during paper making [Citation23]. The abdomen contains several glands: the Dufour’s gland is associated with the female reproductive system [Citation19]. The poison or venom gland is a modified accessory gland to the reproductive system. All female wasps have both Dufour’s and venom glands [Citation21]. The volatile compounds of the venom gland of five Polistes species excluding P. versicolor have been documented [Citation24,Citation25]. The venom of several Polistes species is known to contain the compound N-(3-methylbutyl)acetamide [Citation24] that was found to be attractive to conspecific individuals in field tests in the USA [Citation25].
Although detailed morphological and histological studies of various glands of P. versicolor have been carried out [Citation26–29], nothing is known about the chemical composition of the different glands and body parts of this wasp species. Studies of the chemical ecology and behavior of Polistes could yield attractants (sex or aggregation pheromones) useful for baits or lures for pest control [Citation25]. The objectives of this study were 1) to determine the biochemical composition of Polistes versicolor’s head, thorax, Dufour’s and venom glands and 2) conduct laboratory assays exposing wasps to body part extracts from both sexes to determine if an extract elicited a behavioral response. Results from this work will be useful in the development of baits and the management of this invasive wasp.
Materials and methods
Sample collection
For the biochemical analyses, forager wasps from different nests were collected in the field near the Charles Darwin Research Station (CDRS), Santa Cruz Island, Galápagos (0.742105°S, 90.304280°W) on 30 May 2019, and brought to the laboratory. Each wasp was dissected under a stereomicroscope in double-distilled water. We harvested Dufour’s glands, cleanly separated from the sting apparatus during dissection (n = 3), venom glands (n = 3), heads (n = 3) and thoraxes (n = 3) each extracted in individual tubes containing 0.5 mL of hexane (Sigma Aldrich, New Zealand) (). This was a qualitative analysis to determine the chemical composition of each gland or body part, and as such, only one replicate would have sufficed.
Figure 1. Lateral view of a Polistes versicolor forager showing the position of head, thorax, abdomen, poison glands and the Dufour’s gland. The location and scale of the glands are approximate only. Wasp illustration based on Parent (2000) and the location of glands based on scheme from Jeanne (1996). Illustration by Julia Eloff.
For the behavioral assays and for preparing the body extracts, nests were collected at random close to the CDRS between June 28 and 18 July 2021 and kept in individual containers in the field. Once in the laboratory, nests were kept individually in custom-made nylon rearing boxes (30 × 30 × 30 cm) and the wasps were fed a solution of sugar, banana and hydrolyzed protein. Water was provided ad libitum. The nests were affixed on top of the boxes to keep the wasps flying and active. The food was replaced every 3 days. The temperature inside the room where the nests were kept ranged between 22°C and 29°C. Sample collection and studies took place under permit numbers PC-31-19 and PC-07-21 of the Galápagos National Park Directorate.
Biochemical analyses
One wasp head and two thorax samples dried out in transit and were discarded from analyses. Samples were filtered, the extracts transferred into new vials and left to dry by evaporation. The extracts were then re-dissolved in 300 µL of hexane. The sample extracts were analyzed using a Shimadzu QP2010 Plus gas chromatograph mass spectrometer (GC/MS, Kyoto, Japan). The oven temperature was initially set at 40°C, maintained for 4 min, and then increased by 20°C/min to 100°C with a 5 min hold, followed by 5°C/min to 300°C and held for 4 min. Helium was used as a carrier gas with a flow rate of 1.45 mL/min (43.4 cm/s; constant flow). The injector and transfer line temperatures were 280°C and 305°C, respectively. Injections (1 µL) were in splitless mode. Compound detection was carried out using electron impact (EI) mass spectrometry (MS). MS detection was obtained at 70 eV, with ions detected from m/z 42–600 detected every 0.3 s.
Compounds of each gland/body part were annotated by comparing their mass spectra with those found in mass spectral libraries (NIST11 and in-house). We aligned the data from all replicates for each gland or body part by retention time, and considered a compound as a reliable detection if it was present in at least two of the three replicates. In cases where there were only two replicates, the compound had to be present in both replicates, and where only a replicate was available, we report all the compounds that met the criteria for a reasonable annotation (see below). We then arranged a list of detected compounds based on their abundance. For each run’s total ion chromatogram (TIC), the peak area of each compound was expressed as a percentage of the total area given by all the compounds present [Citation24]. Next, the most abundant compounds in each gland or body part were considered a reasonable annotation if their Similarity Index (SI) scores were ≥85 () to those found in the spectral libraries. If two or more compounds had the same SI score, we provide both compound names in . We show each replicate for each gland or body part separately in to highlight inter-individual variation. From the final compound list, we selected the 10 most abundant compounds to confirm identity through comparison of mass spectra and retention times with those of authentic analytical standards. The following analytical standards were acquired from Sigma Aldrich (New Zealand): heneicosane, tetracontane, tetratetracontane, pentatriacontane, oleamide, linolenic acid, ethyl palmitate and ethyl stearate. β-sitosterol and N-(3-methylbutyl)acetamide (MBA) were sourced from Oakwood Chemical (USA). The compound MBA was selected as it was previously detected in large quantities in the venom gland of several other Polistes spp [Citation24,Citation25].
Table 1. Most abundant putative compounds detected by GC/MS in the head, thorax, venom glands and Dufour’s gland of P. versicolor foragers from the Galápagos Islands. Area% corresponds to each compound’s abundance relative to all compounds detected. Compounds with potential annotations SI score ≥85 were considered reasonable. The identity of the compounds in bold was confirmed through comparison of mass spectra and retention times with those of authentic analytical standards. Compounds labeled Unidentified were potential annotations that were invalidated when compared to analytical standards; the potential annotated name between brackets has been left for reference. More than one potential annotation is shown if their SI score was the same, or if the compound’s first annotation identity was not confirmed.
Behavioral responses to body part extracts
Extracts were prepared from 10 male and 10 female wasps collected from three different nests on the ground of the CDRS and previously frozen at –12°C. Three extracts from male and three from female body parts were made. First, the body parts of the 10 male and 10 female wasps were divided by sex and by head, thorax and abdomen to create extracts of each body part. Ten heads were then ground using a mortar and pestle with 5 mL dichloromethane (DCM). When grinding was finished, the head extract was pipetted into a sterile glass vial and the solution brought to 10 mL by adding DCM to a concentration of 0.1 wasp-equivalents per 100 µL, which resulted in each 100 µL of this solution containing 0.1 wasp heads [following 20]. We express concentrations per 100 µL of solvent as this was the dose used in the arena assays (see below). The same procedure was repeated to obtain thorax and abdomen extracts for each sex. The extracts were then stored at –12°C until use. Dissection tools, glassware, mortar and pestle were cleaned in hot water with a cleaning solution, rinsed, air-dried and baked overnight at 150°C after use.
Wasps for the behavioral assays were sourced from wasp nests that were collected using the procedures described above. Nests were maintained in the experimental room for 1.5 to 2 hours prior to the experiment to allow acclimation to the assay conditions. All trials took place between 10:00 and 15:00 under a fluorescent light. The table on which the assays took place was covered in brown butcher paper. Throughout the experimental assays, the mean laboratory temperature was 26.4 ± 1.3°C and the mean relative humidity, 69.4 ± 7.4%.
We simultaneously compared the reaction of four individual female wasps to one of the following treatments (female: solvent control, head, thorax or abdomen extracts or male: solvent control, head, thorax or abdomen extracts) using an arena assay [Citation20]. Each of these blocks was replicated five times in a continuous series with a total of 20 wasp replicates. The same procedure was repeated for male wasps. A total of 320 wasps were tested (160 males and 160 females). The position of the treatments was rotated for each new block with 5 cm between treatments (petri dishes). Each wasp was placed inside a petri dish (9 cm diameter) using sterile, soft tweezers and allowed 4 min to acclimate. Next, a 1 × 1 cm square piece of filter paper (#3 Whatman, Cytiva, USA) treated with 100 µL of DCM control or body part extract (0.1 wasp-equivalents) was introduced into the arena. The behavior of the wasp was observed for 3 min and the following responses to the extract recorded: i) grooming (rubbing legs over the body), ii) antennation of the filter paper, iii) gastral dragging (the posterior gastral sternites are pressed to the substrate while moving), iv) touch and grab the filter paper with the legs and v) no response. Once the 3 minutes was over, the brown butcher paper, the used petri dishes and the wasps used in the assays were immediately removed from the experimental room. The paper and petri dishes were discarded. Wasps were not reused in our trials.
We used 2 × 2 contingency tables to record behavior for each sex to compare the number of times an assay was positive for one of the four behaviors using G-tests to assess the hypothesis that an observed behavior was independent of the body part extract. For example, we ran a G-test to analyze how many times males responded by grooming (or not grooming) when exposed to female head extracts compared to the solvent control, and repeated this analysis for each sex and behavior combination. All 2 × 2 contingency tables analyzed are available in the Supplementary Material. We tested male and female wasps for behavioral responses to the different body part extracts from both sexes. Analyses were conducted using R version 4.1.2 [Citation30] using the “GTest” command of the package DescTools [Citation31] using the chi-square statistic with a significance level of P < 0.05.
Results
Biochemical analyses
The wasp head samples were mainly composed of hydrocarbons and lipids. The most abundant component was 8-hexylpentadecane. Also present in the heads were oleamide, (Z)-7-tetradecenal and hexacosane. Oleamide is a fatty acid amide whose presence in the head was confirmed by comparison with the analytical standard ().
The only thorax sample examined presented a number of hydrocarbons and lipids. The main compound found was (Z)-7-tetradecenal, a lipid. Oleamide was also present in the thorax, and its identity confirmed by comparison with the authentic standard ().
The venom gland’s most abundant compound, present in all three replicates, was ethyl oleate, a fatty acid ester; followed by oleamide, whose identity was confirmed. A third compound also present in all three replicates was β-sitosterol oleate, a sitosterol. Ethyl stearate, another fatty acid ester, was also present in the venom gland and its identity confirmed (). From our analysis, we can confirm that MBA was not present in the venom gland of the P. versicolor foragers examined in this study.
The majority of compounds annotated for the Dufour’s glands were hydrocarbon lipids. A branched saturated hydrocarbon, 3-methyl heptadecane, was the most abundant compound present in only one of the Dufour’s gland replicates, albeit with a high abundance of approximately 30%, which is why we retained this annotation. A branched alkane, 2-methyloctacosane, was present in all replicates, and octacosanol, a fatty alcohol, in two of the three replicates.
It is worth highlighting that the compounds reported for the different body parts and glands correspond to potential annotations, unless specified as identity confirmed. The identity of the compounds annotated needs to be validated through comparison of mass spectra and retention times with those of authentic analytical standards (see legend for more information).
Behavioral responses to body part extracts
Yellow paper wasps that were exposed to body part extracts from the same or the opposite sex either did not respond or responded with one or more of four behaviors: antennation, grooming, gaster dragging, or touching the paper and grabbing it with their legs (, Supplementary Material). Overall, head extracts elicited stronger behavioral responses in male and female wasps than did thorax or abdomen extracts (). Female wasps reacted to extracts from dissected female heads by grooming more often (χ2 = 10.60, df = 1, P = 0.001) compared to the solvent blanks. Females also antennated more often (χ2 = 9.52, df = 1, P = 0.002) and showed more gastral dragging (χ2 = 4.40, df = 1, P = 0.03) when exposed to female thorax extracts, compared to the solvent blanks. Similarly, the female abdomen extracts elicited more gastral dragging by female foragers (χ2 = 7.65, df = 1, P = 0.005) and more touch behavior (χ2 = 4.40, df = 1, P = 0.03) compared to the solvent blanks. Males responded to female head extracts by grooming more often (χ2 = 13.20, df = 1, P = 0.0003), gastral dragging more often (χ2 = 9.38, df = 1, P = 0.002), and by touching the paper more often (χ2 = 4.40, df = 1, P = 0.03) when compared to the blanks. Males also presented gastral dragging more often when exposed to female thorax extracts (χ2 = 9.38, df = 1, P = 0.002) compared to the blanks.
Table 2. Number of positive responses to exposure to filter paper treated with body part extracts from female (top) or male (bottom) P. versicolor wasps in an arena assay. Numbers in bold and marked with * are significantly different from the solvent blanks in the respective 2 × 2 contingency table analysis.
Male extracts, in comparison to female extracts, elicited fewer significant behavioral responses in wasps (). Females exposed to male head extracts groomed more often (χ2 = 4.05, df = 1, P = 0.04) when compared to the solvent blanks. Males reacted by touching the paper more often when exposed to male head extracts (χ2 = 4.40, df = 1, P = 0.03) in comparison to the blanks.
Discussion
This study was the first to determine the biochemical composition of the head, thorax, Dufuor’s and venom glands of P. versicolor. We also examined the behavioral responses of male and female P. versicolor wasps when exposed to body part extracts from the same or the opposite sex and compared these to solvent blanks. Female body extracts consistently elicited more behavioral responses in both male and female wasps than male extracts.
The majority of compounds in the heads of P. versicolor were straight chain saturated hydrocarbons, but we also found branched alkanes, fatty amide lipids and fatty aldehydes. The most abundant component was 8-hexylpentadecane, with 2-methyloctacosane, oleamide, (Z)-7-tetradecenal and hexacosane also present. The compound 2-methyloctacosane, a branched alkane, was previously found in the cuticle of P. versicolor [Citation32], P. dominula [Citation33,Citation34] and in the cuticle of three species of Mischocyttarus [Citation35]. Branched alkanes have been identified as responsible for chemical signaling between individuals [Citation33,Citation34]. Another compound that was found and its identity confirmed in the head, thorax and venom glands of P. versicolor is oleamide, a fatty amide lipid. Oleamide is present in a variety of insects, and it has a known function in the genus Polistes. Oleamide is the preferred ligand for odorant-binding proteins (OBP) in Polistes dominula. Odorant-binding proteins were found in the antennae, legs and wings of P. dominula, with the OBP only binding to oleamide, which is regarded as a “chemical messenger” [Citation5]. However, we did not find any of the compounds annotated for the ectal mandibular glands, located in the head of P. dominula [Citation36], in our study. In the desert locust, oleamide is the natural ligand of chemosensory proteins of the wings [Citation37].
The thorax of P. versicolor presented a mixture of fatty aldehydes, long-chain alkanes and fatty amide lipids. The main compound found, (Z)-7-tetradecenal is a lipid which acts as a sex pheromone in three species of moths in the genus Prays that are pests of citrus and olive trees [Citation38]. Oleamide, 2-methyloctacosane and 8-hexylpentadecane were present in the thorax as well.
The venom gland of P. versicolor was composed of fatty acid esters, fatty amide lipids and sitosterol. The compounds ethyl oleate, oleamide, β-sitosterol oleate and ethyl stearate were annotated. Of these, oleamide and ethyl stearate were confirmed by comparison with authentic standards. Previous studies determined the volatiles of the venom of five Polistes species, observing qualitative and quantitative differences between them [Citation24,Citation39]. The venom volatiles of P. dominula foragers are a complex mixture of acetates of saturated, mono- and di-unsaturated alcohols, spiroacetals and amides [Citation24]. The venom of P. dominula foragers appears to function as alarm pheromones, reducing the threshold for attack and acts as an attractant on targets [Citation40,Citation41]. The compound N-(3-methylbutyl)acetamide (MBA) is common in insect exocrine secretions, and is a principal volatile component in the venom of female vespids, including Polistes [Citation25,Citation42]. We did not detect this compound in the venom glands of P. versicolor. The presence of MBA in the venom is thought to vary depending on the species, sex, caste and context [Citation25].
The Dufour’s glands of P. versicolor are composed of a mixture of hydrocarbon lipids and fatty alcohols, as has been found for P. dominula [Citation43]. The compound 3-methyl heptadecane was the main component in one of the three replicates, with high abundance. Heptadecane was reported to be the main component of a pheromone in arctiid moths in the genus Holomelina [Citation44], and it is also present in the genital chamber of a scarab beetle, Phyllophaga opaca [Citation45]. A branched alkane, 2-methyloctacosane, was present in all Dufour’s glands examined. This compound is also present in the cuticle of P. versicolor [Citation32] as explained above. Previously, Dani et al. [Citation43] reported that the secretion of the Dufour’s gland of P. dominula contains the same hydrocarbons which occur in the cuticle, and this seems to be the case for P. versicolor as well. The Dufour’s gland secretion is involved in nestmate and conspecific recognition in P. dominula [Citation46] and in dominance interactions in P. fuscatus [Citation47,Citation48].
Female yellow paper wasps when exposed to female head extracts displayed more grooming than expected; when exposed to female thorax extracts showed more antennation and gastral dragging than expected; and when exposed to female abdomen extracts displayed more gastral dragging and more touch behavior than expected. Male yellow paper wasps when exposed to head extracts from females produced significantly more grooming, more gastral dragging and more touch compared to the solvent blanks. Males also showed more gastral dragging when presented with thorax extracts from females. Only male head extracts elicited a significant response from females (more grooming) and from males (more touch). However, we suspect that this touch behavior might be significant by chance and an increased sample size would be needed to confirm this result.
There was a trend for the head extracts to elicit significantly more behavioral responses in female and male wasps. There are eight pairs of glands in the female head, mostly associated with the mouthparts and oral cavity. Landolt and Akre [Citation19] suggested that the secretions of some of these gnathal glands may be pheromones distributed by trophallaxis between adults, fed to larvae or added to nest materials. Foragers are the ones involved in larval feeding and nest building, so it would make sense that some of the secretions from foragers have messages for other foragers. It is also conceivable that the secretions of certain glands change with the colony cycle, caste or sex. It is known that the ectal mandibular glands of male P. fuscatus produce a sex pheromone, while the same glands in foragers and queens have a different function [Citation19]. We used whole head extracts in our study as our aim was to find compounds that may result attractive to these wasps, not to determine gland function.
Males scent-marked (dragged the sternites of the gasters on filter paper) in response to head and thorax extracts from females, as seen in P. exclamans in another study [Citation20]. In Polistes, males dragging the gastral sternites on a substrate is considered to involve the deposition of pheromones [Citation20,Citation49–51], possibly to mark territory and attract potential mates. Unmated females of P. exclamans are attracted to sex pheromones released from the mandibular and sternal glands of males [Citation52]. Gaster dragging has been documented in perching and patrolling males of various Polistes species [Citation51] and is thought to be a scent-marking behavior [Citation52]. It remains to be determined if pheromones are also involved in male-female attraction in P. versicolor.
Conclusions
Wasp semiochemicals pose an alternative to non-specific baits commonly used for controlling pestiferous wasps [Citation16] and are under exploration for controlling other Polistes species. For example, the compound MBA, found in the venom of Polistes metricus, P. bellicosus, P. aurifer and P. dorsalis, attracted conspecifics in field tests in the USA [Citation25]. MBA, however, does not appear to have the potential for use in the control of P. versicolor as this was not found in the venom glands. Perhaps of greater promise are semiochemicals found in the heads and thorax of female P. versicolor wasps, which produced a behavioral reaction in other yellow paper wasps. The next logical step is to run laboratory and field trials with different compound blends to evaluate their effectiveness as attractants for P. versicolor. The end goal is to develop a lure and kill method to reduce wasp numbers in human-inhabited areas (tourist visitor sites, fruit and vegetable markets, farms) and in areas of high conservation value.
Supplemental Material
Download MS Excel (25.1 KB)Acknowledgments
We thank the Galápagos National Park Directorate for issuing research permits and for field and logistical support, in particular, Danny Rueda and Christian Sevilla; Christine Parent for helping with sample transport; Heinke Jäger for help in accessing materials and logistical support; and special thanks to Julia Eloff for producing the paper wasp illustration. We are extremely grateful to the two anonymous reviewers. Reviewer 1 is especially thanked for a detailed and critical reading, and for providing constructive advice to improve the original manuscript. This study was supported by a grant from the Galapagos Conservancy to PJL and MB and a grant from Lindblad Expeditions-National Geographic Fund to CEC. This publication is contribution number 2428 of the Charles Darwin Foundation for the Galapagos Islands.
Disclosure statement
The authors declare no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/23766808.2022.2098575
Additional information
Funding
References
- Beggs JR, Brockerhoff EG, Corley JC, et al. Ecological effects and management of invasive alien Vespidae. BioControl. 2011;56:505–526.
- Kenis M, Auger-Rozenberg M-A, Roques A, et al. Ecological effects of invasive alien insects. Biol Invasions. 2009;11:21–45.
- Moller H. Lessons for invasion theory from social insects. Biol Conserv. 1996;78:125–142.
- Lorenzi MC, Bagneres A-G, Clement J-L. The role of cuticular hydrocarbons in social insects: is it the same in paper-wasps? In: Turillazzi S, West-Eberhard MJ, editors. Natural history and evolution of paper wasps. New York: Oxford University Press; 1996. p. 178–189.
- Calvello M, Guerra N, Brandazza A, et al. Soluble proteins of chemical communication in the social wasp Polistes dominulus. Cell Mol Life Sci. 2003;60:1933–1943.
- Gamboa GJ. Sister, aunt-niece, and cousin recognition by social wasps. Behav Genet. 1988;18:409–423.
- Gamboa GJ. Kin recognition in eusocial wasps. Ann Zool Fenn. 2004;41:789–808.
- Jeanne RL. The evolution of exocrine gland function in wasps. In: Turillazzi S, West-Eberhard MJ, editors. Natural history and evolution of paper wasps. New York: Oxford University Press; 1996. p. 144–160.
- Cervo R, Zacchi F, Turillazzi S. Polistes dominulus (Hymenoptera, Vespidae) invading North America: some hypotheses for its rapid spread. Insectes Soc. 2000;47:155–157.
- Howse MWF, Haywood J, Lester PJ. Bioclimatic modelling identifies suitable habitat for the establishment of the invasive European paper wasp (Hymenoptera: Vespidae) across the Southern Hemisphere. Insects. 2020;11:784.
- McGruddy R, Howse MWF, Haywood J, et al. Nesting ecology and colony survival of two invasive Polistes wasps (Hymenoptera: Vespidae) in New Zealand. Environ Entomol. 2021;50:1466–1473.
- Parent CE, Peck SB, Causton CE, et al. Polistes versicolor (Hymenoptera: Vespidae), an introduced wasp in the Galapagos Islands: its life cycle and ecological impact. Environ Entomol. 2020;49:1480–1491.
- Richards OW. The social wasps of the Americas excluding the vespinae. London, UK: British Museum (Natural History); 1978.
- Causton CE, Peck SB, Sinclair BJ, et al. Alien insects: threats and implications for conservation of Galapagos Islands. Ann Entomol Soc Am. 2006;99:121–143.
- Grosch J, Hilger C, Bilo MB, et al. Shedding light on the venom proteomes of the allergy-relevant Hymenoptera Polistes dominula (European Paper Wasp) and Vespula spp. (Yellow Jacket). Toxins (Basel). 2020;12(5):323.
- Landolt P, Zhang QH. Discovery and development of chemical attractants used to trap pestiferous social wasps (Hymenoptera: Vespidae). J Chem Ecol. 2016;42:655–665.
- Landolt PJ. A chemical attractant for the Golden Paper Wasp, Polistes aurifer Saussure (Hymenoptera: Vespidae). J Kansas Entomol Soc. 1998;71:69–73.
- Lasso-Vaca MT. Ecología e impacto de la avispa introducida (Polistes versicolor, Vespidae-Hymenoptera) en las islas Floreana y Santa Cruz. Galápagos, Ecuador. Quito, Ecuador: Pontificia Universidad Católica de Ecuador; 1997.
- Landolt PJ, Akre RD. Occurrence and location of exocrine glands in some social Vespidae (Hymenoptera). Ann Entomol Soc Am. 1979;72:141–148.
- Elmquist DC, Landolt PJ, Ream LJ, et al. Laboratory demonstrations of pheromone-mediated scent-marking, orientation, and mounting behavior in Polistes exclamans (Hymenoptera: Vespidae). Ann Entomol Soc Am. 2018;111:21–30.
- Downing HA. The function and evolution of exocrine glands. In: Ross KG, Matthews RW, editors. The social biology of wasps. New York: Cornell University Press; 1991. p. 540–569.
- Akre RD, Garnett WB, Donald JFM, et al. Behavior and colony development of Vespula pensylvanica and V. atropilosa (Hymenoptera: Vespidae). J Kansas Entomol Soc. 1976;49:63–84.
- Spradbery JP. An account of the biology and natural history of solitary and social wasps. Seattle: University of Washington Press; 1973.
- Bruschini C, Dani FR, Pieraccini G, et al. Volatiles from the venom of five species of paper wasps (Polistes dominulus, P. gallicus, P. nimphus, P. sulcifer and P. olivaceus). Toxicon. 2006;47:812–825.
- Elmquist DC, Landolt PJ, Cooper WR, et al. The venom compound N-(3-methylbutyl)acetamide attracts several Polistes (Fuscopolistes) species (Hymenoptera: Vespidae). J Econ Entomol. 2020;113:1073–1079.
- Brito JHdS, Antonialli-Junior WF, Montagna TDS, et al. Linear alkanes and reproductive status of Polistes versicolor (Hymenoptera: Vespidae) females in winter aggregates. Sociobiol. 2017;64:327–333.
- Britto FB, Caetano FH. Ultramorphological analysis of the venom glands and their histochemical relationship with the convoluted glands in the primitive social paper wasp Polistes versicolor (Hymenoptera: Vespidae). J Venom Anim Toxins includ Trop Dis. 2005;11:160–174.
- Britto FB, Caetano FH. Morphological features and occurrence of degenerative characteristics in the hypopharyngeal glands of the paper wasp Polistes versicolor (Olivier) (Hymenoptera: Vespidae). Micron. 2006;37:742–747.
- Britto FB, Caetano FH. Ultrastructural features of the hypopharyngeal glands in the social wasp Polistes versicolor (Hymenoptera: Vespidae). Insect Sci. 2008;15:277–284.
- R Core Team. R: a language and environment for statistical computing. 4.1.2. Vienna, Austria: R Foundation for Statistical Computing; 2021.
- Signorell A, Aho K, Alfons A, et al. DescTools: tools for Descriptive Statistics. R package version 0.99.45. 2022.
- Michelutti KB, Soares ERP, Sguarizi-Antonio D, et al. Influence of temperature on survival and cuticular chemical profile of social wasps. J Therm Biol. 2018;71:221–231.
- Dani FR, Foster KR, Zacchi F, et al. Can cuticular lipids provide sufficient information for within-colony nepotism in wasps? Proc Royal Soc B. 2004;271:745–753.
- Dani FR, Jones GR, Destri S, et al. Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Anim Behav. 2001;62:165–171.
- Soares ERP, Batista NR, RdS S, et al. Variation of cuticular chemical compounds in three species of Mischocyttarus (Hymenoptera: Vespidae) eusocial wasps. Rev Bras Entomol. 2017;61:224–231.
- Fortunato A, Maile R, Turillazzi S, et al. Defensive role of secretion of ectal mandibular glands of the wasp Polistes dominulus. J Chem Ecol. 2001;27:569–579.
- Tomaselli S, Crescenzi O, Sanfelice D, et al. Solution structure of a chemosensory protein from the desert locust Schistocerca gregaria. Biochem. 2006;45:10606–10613.
- Gibb AR, Jamieson LE, Suckling DM, et al. Sex pheromone of the citrus flower moth Prays nephelomima: pheromone identification, field trapping trials, and phenology. J Chem Ecol. 2005;31:1633–1644.
- Bruschini C, Cervo R, Protti I, et al. Caste differences in venom volatiles and their effect on alarm behaviour in the paper wasp Polistes dominulus (Christ). J Exp Biol. 2008;211:2442–2449.
- Bruschini C, Cervo R. Venom volatiles of the paper wasp social parasite Polistes sulcifer elicit intra-colonial aggression on the nest of the host species Polistes dominulus. Insectes Soc. 2011;58:383–390.
- Bruschini C, Cervo R, Turillazzi S. Evidence of alarm pheromones in the venom of Polistes dominulus workers (Hymenoptera: Vespidae). Physiol Entomol. 2006;31:286–293.
- El-Sayed AM, Brown RL, Bunn B. N-(3-methylbutyl)butanamide: a novel amide in the venom of female social wasps, Vespula vulgaris. J Insect Physiol. 2021;135:104311.
- Dani FR, Morgan ED, Turillazzi S. Dufour gland secretion of Polistes wasp: chemical composition and possible involvement in nestmate recognition (Hymenoptera: Vespidae). J Insect Physiol. 1996;42:541–548.
- Charlton RE, Roelofs WL. Biosynthesis of a volatile, methyl-branched hydrocarbon sex pheromone from leucine by arctiid moths (Holomelina spp.). Arch Insect Biochem Physiol. 1991;18:81–97.
- Romero-López AA, Reyes-Chilpa R, Pérez-Flores FJ, et al. Chemicals in the genital chamber of two Mexican species of Phyllophaga. Southwest Entomol. 2019;44:457–464.
- Dani FR, Fratini S, Turillazzi S. Behavioural evidence for the involvement of Dufour’s gland secretion in nestmate recognition in the social wasp Polistes dominulus (Hymenoptera: Vespidae). Behav Ecol Sociobiol. 1996;38:311–319.
- Downing HA. A role of the Dufour’s gland in the dominance interactions of the paper wasp, Polistes fuscatus (Hymenoptera: Vespidae). J Insect Behav. 1991;4:557–565.
- Downing HA, Jeanne RL. Correlation of season and dominance status with activity of exocrine glands in Polistes fuscatus (Hymenoptera: Vespidae). J Kansas Entomol Soc. 1983;56:387–397.
- Beani L, Calloni C. Leg tegumental glands and male rubbing behavior at leks in Polistes dominulus (Hymenoptera: Vespidae). J Insect Behav. 1991;4:449–462.
- Beani L, Calloni C. Male rubbing behaviour and the hypothesis of pheromonal release in polistine wasps (Hymenoptera Vespidae). Ethol Ecol Evol. 1991;3(sup1):51–54.
- Wenzel JW. Male reproductive behavior and mandibular glands in Polistes major (Hymenoptera: Vespidae). Insectes Soc. 1987;34:44–57.
- Reed HC, Landolt P. Sex attraction in paper wasp Polistes exclamans Viereck (Hymenoptera: Vespidae), in a wind tunnel. J Chem Ecol. 1990;16:1277–1287.
