ABSTRACT
The habitat fragmentation caused by reservoir construction in different aquatic systems in the Magdalena River basin is a latent threat to the diversity of fishes and aquatic environments in the Andean region of Colombia. Contributing to the knowledge about how fish assemblages are formed in these scenarios is fundamental for the management and decision-making on the aquatic resources of the basin. In this communication, we describe the results of a fish characterization in the area of influence of the Piedras Blancas reservoir, a high Andean reservoir built for hydroelectric power generation. Only three species of fish that make up the evaluated assemblage were captured, and two of them, Cyprinus carpio and Xiphophorus maculatus, are not native to the basin. Possibly, with a greater sampling effort, more species can be found. The native species Hemibrycon sp. is distributed mainly in a creek, while in the reservoir, the introduced species dominate. The environmental variables were in optimal ranges for the development of life in the evaluated environments. It is important to advance the knowledge about the distribution of the introduced and native species in the basin to generate recommendations that contribute to the management of these species in the aquatic systems of Colombian Andes.
The development of the hydroelectric power generation industry in the Magdalena-Cauca basin has led to the construction of reservoirs in different rivers and elevations throughout the basin [Citation1]. Habitat fragmentation caused by the dam creates new aquatic environments by forming a new artificial system, the reservoir [Citation2]. This new system isolates small bodies of water located within its area of influence, and it changes fish distribution [Citation2,Citation3]. Most native fish species do not present the physiological, morphological, and behavioral conditions to remain in these modified aquatic systems, so reproduction and recruitment falls [Citation4]; this leads to a decline in their abundances and in the alpha diversity [Citation2,Citation4]. The new habitat conditions caused by fragmentation favor new fish species establishment, mainly non-native to the basin, as they have the physiological conditions and feeding and reproductive strategies to occupy these modified aquatic environments [Citation2,Citation5]. These fish species are introduced with the purpose of promoting the fishing resource, sport fishing, or in some cases, the occasional release of fish species used as ornamentals due to undesirable characteristics such as large body size, high reproductive output, and aggressive behavior [Citation5].
In the reservoirs of the Magdalena River basin, there are about 11 species of fish that have been introduced in these lake systems; Cichlids are the most frequent [Citation6]. The presence of these non-native species is influenced by the elevation and associated environmental conditions where the reservoir was built. This indicates that the environmental characteristics for its occurrence are similar in both its native and invasive range [Citation7]. For example, cichlids originating from tropical Africa thrive in warm water environments; in the Magdalena River basin, these environmental conditions can be found between 100 and 900 m elevation (water temperature between 25 and 28 °C) [Citation8]. While species of temperate origin, such as the genera Cyprinus, Micropterus and Oncorhynchus, prefer colder waters, similar characteristics occur in the basin from 1800 m elevation (water temperature between 16 and 21 °C) [Citation8].
In the area of influence of reservoirs built in the highlands of the basin (higher than 2000 m elevation), it has been detected that non-native species of the genera Cyprinus and Micropterus dominate in the reservoirs, while in streams, the assemblage is mainly composed of native species of the genera Hemibrycon and Trichomycterus [Citation9]. Otherwise, in the reservoirs in the Porce River, located between 540 and 900 m elevation, Cichlids species are abundant [Citation10]. Because the transformation of the habitat caused by the dam construction exerts a high pressure on aquatic biota communities, it is important to provide knowledge on the distribution of the endemic Andean ichthyofauna to habitat fragmentation. It contributes to taking proper decisions about management and conservation of aquatic systems in the Magdalena River basin [Citation11]. This communication presents the results of a characterization of fish diversity in the area of influence of the Piedras Blancas reservoir, as a management action in the reservoirs in the Magdalena River basin.
The Piedras Blancas reservoir is located on the eastern margin of the central mountain range of the Colombian Andes, east of the city of Medellín in the department of Antioquia (06°17“ to 06°18” North; 75°29“ to 75°30” West), and covers an area of ~28.8 km2 (). This highland region has altitudes between 2200 and 2600 (m a.s.l) and an average annual environmental temperature of 14.7 °C [Citation12]. Two rainy seasons are reported in the year (April-May and September-November) and present an average rainfall of 910 mm/season [Citation12]. This hydroelectric power generation plant is managed by Empresas Públicas de Medellín and began its operation in 1958. The tributaries to the reservoir are the Piedras Blancas and Chorrillos creeks [Citation12].
Figure 1. Map of the Piedras Blancas reservoir in the Magdalena River basin and sampling sites. E1, reservoir; E2, reservoir near dam; Q1, Chorrillos Creek; Q2, Piedras Blancas Creek.
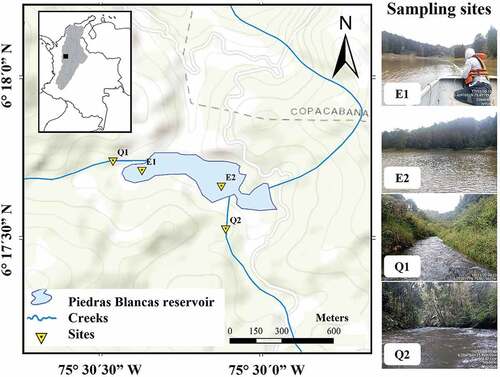
A field sampling trip was carried out between the 16th and 18th of November, in the year 2020. The samples were taken at four sites (). In the field, the sampling site locations were georeferenced (Garmin GPSMAP 64s), and a 100 -m long section was selected for fish capture. Electrical conductivity µS/cm, water temperature °C, dissolved oxygen mg/L, percent oxygen saturation (%), and pH were recorded using a portable multiparameter (Hach HQ40d; https://co.hach.com), and the transparency of the water (m) was measured using a Secchi disk (Table S1).
The fish catches were made between 06:00 and 18:00 hours. In the creeks, portable electric fishing equipment was used with one ampere of pulsating current (340 V, 1–2 A, dc), and as a complement, cast nets of different mesh sizes were used (0. 5 and 2 cm, full mesh). Stationary nets 100 m long and 3 m high were used in the reservoir, with different mesh sizes (1–10 cm) to increase the probability of capturing different species. The captured specimens were deposited in containers with portable aerators to protect their integrity. Representatives of the collected species were anesthetized in clove solution [Citation13] and fixed in formalin at a 10% concentration for their taxonomic identification in the laboratory. Each specimen was identified to the species level by using specialized taxonomic keys [Citation5,Citation14,Citation15], and its name was checked against Fricke et al. [Citation16] for validation. The collected material was deposited in the Ichthyological Collection of the Universidad de Antioquia (CIUA-168).
Fish species diversity was calculated using the effective number of species or Hill numbers (q0, q1 and q2) [Citation17], following the method proposed by Jost [Citation18] and Chao et al. [Citation19]. The first-order richness or effective richness is when q = 0, which represents a condition in which species are present with at least one individual, and therefore, only detected species contribute to the estimate of diversity. The effective richness when q = 1 is undefined; however, the limit of q tends to one and is equivalent to the Shannon diversity exponent. The q1 estimator is highly sensitive to the frequency of species with low or medium abundance (common species). For q = 2, diversity is equivalent to the inverse of Simpson’s index, which is sensitive to the presence of dominant species [Citation18]. To compare alpha diversity and assess sampling representativeness among different aquatic environments, rarefaction curves based on the number of individuals and interpolation/extrapolation of Hill numbers were used, with a confidence interval constructed using the bootstrap resampling method [Citation19], implemented in the iNEXT package [Citation20]. When the extrapolation curve reaches the asymptote, it allows estimating the proportion of species that the current sampling managed to record with respect to a potential total richness indicated by the asymptote, where values close to one indicate robust diversity sampling [Citation19].
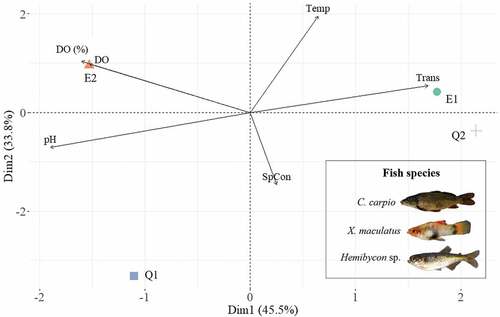
In addition, to describe the influence of the physicochemical variables associated with the sampling sites, we conducted principal component analysis (PCA), using the data containing the description of the physicochemical characteristics of the water body in each sampling site; variable values were centered and scaled to reduce the effect of the measurement units in the PCA analysis. This analysis was performed using the dudi.pca function from the ade4 package [Citation21]. All statistical and graphical analyses were performed using R software (version 3.6.3).
A total of 83 individuals representing three species, three families, and three orders were captured ( and Table S2). Two species are not native to the basin: the common carp Cyprinus carpio Linnaeus 1758 and the guppy Xiphophorus maculatus (Günther 1866). The highest captures were recorded in the Chorrillos Creek (Q1) (n = 63) and in a lower number in the reservoir sites (n = 20) (Table S2). No individuals were captured in the Piedras Blancas Creek (Q2). The most abundant species was Hemibrycon sp. (n = 77), followed by X. maculatus (n = 4) and C. carpio (n = 2). In the reservoir, the fish assemblage is made up of the three species caught, while in the Chorrillos Creek the assemblage consisted of a single species (Hemibrycon sp) (Table S2).
Figure 2. Rarefaction curves based on the sample size (solid lines) and extrapolation (dashed lines) of fish species diversity based on Hill numbers (q = 0, 1, 2).
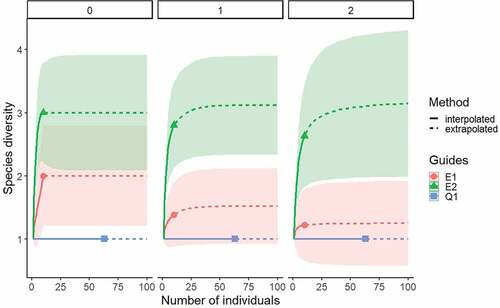
Fish richness according to the effective number of species of order q = 0 was higher in the reservoir sites ( and Table S3). On the other hand, common (order q = 1) and dominant (order q = 2) species were similar in the reservoir sites and were lower in Chorrillos Creek ( and Table S3). There were no differences in diversity values between sampling sites with a statistical confidence interval of 95 % (Table S3). The expected sample coverage indicates incomplete sampling, since it is possible to capture more species in the aquatic environments evaluated (Table S3).
The PCA shows that the first two principal components explain 79.3% of the total accumulated variance (). The physicochemical variables such as dissolved oxygen, oxygen saturation percentage, transparency, and temperature were associated with the reservoir sites ( and Table S4), while in the creek sites, an association was observed in the variables that presented high values of conductivity and pH ( and Table S4).
Figure 3. Principal Component Analysis of physicochemical variables and sampling sites. Temp, temperature (° C); SpCon, specific conductivity (µS/cm); DO, dissolved oxygen (mg/L); DO, Percent Oxygen Saturation (%); Trans, transparency (m) and pH.
Contribution to the knowledge of the fish diversity associated with impounded water bodies and introduced species is a necessary tool for implementing biodiversity management actions in the Magdalena River basin [Citation5,Citation22]. In this study, we report the occurrence of three species in the area of influence of the Piedras Blancas reservoir, and two of them have been introduced. The number of species captured was low; possibly, with a greater sampling effort we would expect to find more species [Citation23]. The species collected coincide with what was found in other investigations that have been carried out in other reservoir systems in the highlands in the Colombian Andes, where it is recorded that native species are distributed in streams, while in lentic systems (that is, reservoir), non-native species dominate [Citation1,Citation9].
The elevation at which this reservoir was built and its associated physicochemical conditions seem to favor the presence of the species C. carpio and X. maculatus, as has been reported in other reservoirs built in the Magdalena River basin [Citation6,Citation9]. Both species are characterized by their high fecundity, a generalist feeding strategy, early sexual development, and their broad environmental tolerance, which facilitates their dispersal and makes them particularly successful as invasive species [Citation5]. On the other hand, in the tributary streams, Hemibrycon sp. individuals were dominant, whose ecological aspects of feeding, reproduction, and morphology allow them to inhabit and carry out their natural life history in these high Andean aquatic systems [Citation15,Citation24,Citation25].
It has been documented that the introduction of non-native fish species is increasingly recognized as an important contributor to extinction risk of local species [Citation26,Citation27]. There are several major ecological effects associated with fish introductions, including predation, increased competition for resources, hybridization and disease transmission, resulting in possible detrimental interactions with native species or even on ecosystem functioning [Citation26,Citation27]. However, adverse ecological effects have been documented for a minority of these species, and management actions may be necessary to minimize their dispersal and impacts [Citation28]. In the Magdalena River basin, the evidence that supports this statement is still scarce [Citation5,Citation29], and therefore, it is important to advance the knowledge of non-native and native species interactions, with approaches in the prediction by competition in conditions of habitat for their life development [Citation7], in addition to their feeding competence [Citation30], thus allowing one to complement the knowledge and propose recommendations that contribute to the management of these species in the aquatic systems of the Colombian Andes.
Supplemental Material
Download MS Word (23.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/23766808.2022.2104971
Additional information
Funding
References
- Jiménez Segura L, Restrepo Santamaría D, López Casas S, et al. Ictiofauna y desarrollo del sector hidroeléctrico en la cuenca del río Magdalena - Cauca, Colombia. Biota Colomb. 2014;15:3–25.
- Valencia-Rodríguez D, Herrera-Pérez J, Restrepo-Santamaría D, et al. Fish community turnover in a dammed Andean River over time. Neotrop Ichthyol. 2022;20:1–19.
- Agostinho AA, Gomes LC, Santos NCL, et al. Fish assemblages in Neotropical reservoirs: colonization patterns, impacts and management. Fish Res. [Internet]. 2016;173:26–36. DOI:10.1016/j.fishres.2015.04.006.
- Arantes CC, Fitzgerald DB, Hoeinghaus DJ, et al. Impacts of hydroelectric dams on fishes and fisheries in tropical rivers through the lens of functional traits. Curr Opin Environ Sustain. [Internet]. 2019;37:28–40. DOI:10.1016/j.cosust.2019.04.009.
- Lasso CA, Escobar MD, Herrera J, et al. Peces introducidos en el río magdalena y cuencas vecinas, Colombia. In: Jiménez-Segura L, Lasso C, editors. Peces la cuenca del río Magdalena, Colomb Divers Conserv y uso Sosten. Bogotá (DC): IAvH; 2020. p. 295–367.
- Jiménez-Segura LF, Alvarez-Leon R, Gutierrez-Bonilla F, et al. La pesca y los recursos pesqueros en los embalses colombianos. In: Lasso CA, Gutiérrez F, Morales-Betancourt M, et al., editors. Pesq Cont Colomb cuencas del Magdalena-Cauca, Sinú, Canalete, Atrato, Orinoco, Amaz y vertiente del Pacífico. [Internet]. Bogotá (DC) Colombia: Instituto de Investigacion de Recursos Biologicos Alexander von Humboldt (IAVH); 2011. p. 233–282. Available from: http://hdl.handle.net/20.500.11761/9332
- Castellanos-Mejía MC, Herrera J, Noguera-Urbano EA, et al. Potential distribution in Colombia of the introduced fish pangasianodon hypophthalmus (Siluriformes: pangasiidae) and implications for endangered native fish. Rev Biol Trop. 2021;69:573–587.
- Herrera-Pérez J, Parra JL, Restrepo-Santamaría D, et al. The influence of abiotic environment and connectivity on the distribution of diversity in an Andean fish fluvial network. Front Environ Sci. 2019;7. DOI:10.3389/fenvs.2019.00009.
- Martinéz Toro L, Restrepo Santamaria D, Valencia Rodríguez D, et al. Ensamblajes de peces en embalses altoandinos: el caso de los embalses Quebradona y Riogrande II en la cuenca Magdalena. Caldasia. [Internet]. 2021;43. DOI:10.15446/caldasia.v44n2.82464.
- Álvarez-Bustamante J, Jaramillo-Villa Ú, Jiménez-Segura LF. Ictiofauna de embalses en cascada en el cauce de un río tropical andino. Actual Biológicas. 2018;40:46–58.
- Jiménez-Segura L, Galvis-Vergara G, Cala-Cala P, et al. Freshwater fish faunas, habitats and conservation challenges in the Caribbean river basins of north-Western South America. J Fish Biol. 2016;89:65–101.
- Roldan Pérez G, Gutiérrez J, Castaño R, et al. Caracterización limnológica de los recursos hidricos del parque de Piedras Blancas [Internet]. Medellín; 1997. Available from: https://www.corantioquia.gov.co/ciadoc/AGUA/AIRNR_CN_113_1995.pdf.
- Javahery S, Nekoubin H, Moradlu AH. Effect of anaesthesia with clove oil in fish (review). Fish Physiol Biochem. 2012;38:1545–1552.
- Maldonado-Ocampo A, Ortega-Lara JS, Usma Oviedo G, et al. Peces De Los Andes De Colombia Colombia. Diversa Por Naturaleza. [Internet]. 2005. Available from: http://awsassets.panda.org/downloads/peces_de_los_andes_de_colombia.pdf
- Román-Valencia C, Arcila-Mesa DK. Five new species of Hemibrycon (Characiformes: characidae) from the Río Magdalena basin, Colombia. Rev Biol Trop. 2010;58:339–356.
- Fricke R, Eschmeyer WN, Van der Laan R. Eschmeyer’s catalog of fishes: genera, species, references. [Internet]. 2021 [cited 2021 Aug 15]. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
- Hill MO. Diversity and evenness: a unifying notation and its consequences. Ecology. 1973;54:427–432.
- Jost L. Entropy and diversity. Oikos. [Internet]. 2006;113:363–375. DOI:10.1111/j.2006.0030-1299.14714.x.
- Chao A, Gotelli NJ, Hsieh TC, et al. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr. [Internet]. 2014;84:45–67. DOI:10.1890/13-0133.1.
- Hsieh TC, Ma KH, Chao A. iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). McInerny G editor Methods Ecol Evol. [Internet]. 2016;7:1451–1456. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/2041-210X.12613
- Thioulouse J, Dray S. Interactive multivariate data analysis in R with the ade4 and ade4TkGUI packages. J Stat Softw. 2007;22:1–14.
- DoNascimiento C, Herrera-Collazos EE, Herrera-R GA, et al. Checklist of the freshwater fishes of Colombia: a Darwin core alternative to the updating problem. Zookeys. 2017;2017:25–138.
- Restrepo-Santamaria D, Herrera-Pérez J, Muñoz-Duque S, et al. Inventarios de peces en la cuenca del río Magdalena como herramienta para la gestión de su conservación. Caldasia. 2022;44:356–367.
- Román-Valencia C, Ruiz-C RI, Giraldo A. Dieta y reproducción de dos especies sintópicas: hemibrycon boquiae y Bryconamericus caucanus (Pisces: characidae) en la quebrada Boquía, río Quindío, Alto Cauca, Colombia. Rev del Mus Argentino Ciencias Nat Nueva Ser. 2008;55–62. DOI:10.22179/REVMACN.10.292
- Román-Valencia C, Arcila-Mesa DK, Hurtado TH. Variación morfológica de los peces Hemibrycon boquiae y Hemibrycon rafaelense (Characiformes: characidae) en el Río Cauca, Colombia. Rev Biol Trop. 2009;57:541–556.
- Gozlan RE, Britton JR, Cowx I, et al. Current knowledge on non-native freshwater fish introductions. J Fish Biol. 2010;76:751–786.
- Cucherousset J, Olden JD. Feature: Introduced fish and ecology. Ecological impacts of non-native freshwater fishes. Fish Bethesda. [Internet]. 2011;36:215–230. Available from: http://www.fish.washington.edu/research/oldenlab/pdf/2011/Fisheries_2011c_Inv.pdf
- Britton JR, Gozlan RE, Copp GH. Managing non-native fish in the environment. Fish Fish. 2011;12:256–274.
- López-Casas S, Rondón-Martínez YF, and Gutiérrez-Cortés A. Diagnóstico del grado de amenaza y medidas de manejo para los peces del río Magdalena, Colombia Peces de la cuenca del río Magdalena, Colombia: diversidad, conservación y uso sostenible . In: Jimenez-segura LF, Lasso CA, Bogotá DC, et al., editors. Colombia: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt. 2020. p. 391–429.
- Córdova-Tapia F, Contreras M, Zambrano L. Trophic niche overlap between native and non-native fishes. Hydrobiologia. 2015;746:291–301.
