ABSTRACT
The presence of a predator can cause behavioral changes in its prey, such as decreased foraging activity. Previous studies have indicated that the scorpion Ananteris mauryi has the ability to identify the chemical traces of its intraguild predator Tityus pusillus, causing it to reduce its exploratory activity. In this study, the foraging activities of A. mauryi in a natural environment with different densities of T. pusillus were compared. The study was carried out through active searches from 19:00 to 22:00 in five fragments of the Atlantic Forest in Pernambuco, Brazil, one having a low density of T. pusillus and four returning high densities. In total, 81 individuals of A. mauryi were observed, and 49.4% were recorded in the area with a low density of T. pusillus. In general, A. mauryi individuals exhibited a similar foraging activity in the fragment with low and higher density of T. pusillus. Similarly, A. mauryi was found on leaf litter bottom layers independently of T. pusillus density. Therefore, our results suggest that their predator density does not exert any influence on foraging activity and microhabitat choice in A. mauryi.
Introduction
Predator–prey interactions are commonly divided into two types: (1) lethal interactions, in which predators consume their prey, and (2) non-lethal interactions, in which the risk of predation generates behavioral changes in prey [Citation1–3]. These mechanisms may interact in synergy depending on the prey-predator features, such as predator hunting mode and predator-prey habitat [Citation4–6]. For example, two buthid scorpion species, Tityus pusillus Pocock, 1893, a sit-and-wait hunter, and Ananteris mauryi Lourenço, 1982 an active hunter, are capable of altering their behavior in the presence of each other [Citation7,Citation8]. Tityus pusillus shifted from its sit-and-wait strategy to actively hunting in the presence of A. mauryi [Citation8]. However, A. mauryi became more vigilant, reducing its frequency of rest. According to Schmitz [Citation9], less active predators, such as sit-and-wait hunters, typically cause stronger antipredator responses because their cues are more persistent in the environment than more active predators.
A common event in nature involves intraguild predation (IGP) where predators compete for similar prey and shelter [Citation10,Citation11]. The relationship between intraguild predators may affect several of their ecological interactions, such as population dynamics and community structure [Citation10,Citation12–16]. Therefore, research on behavioral IGP interactions provides important insights for behavioral and ecological studies.
In this context, scorpions may be good models for studies investigating the interactions in an IGP system, particularly since these arachnids are primarily solitary and prefer to inhabit areas close to their prey [Citation17]. IGP generally occurs in this group when smaller species are preyed upon by larger ones [Citation13,Citation18,Citation19]; therefore, the smaller species likely developed mechanisms to co-exist with their larger counterparts [Citation20]. For example, previous studies have indicated that smaller species are active in different time periods than larger scorpions, or they change their foraging sites to avoid predation [Citation8,Citation13,Citation21].
In a previous study, we characterized scorpion microhabitats in the rainy and dry seasons in the Brazilian Atlantic Rainforest and Caatinga Dry Forest [Citation22]. Expanding our analysis, we assessed how the density of T. pusillus (intraguild predator) may affect foraging behavior and microhabitat use of A. mauryi (intraguild prey). These scorpions co-exist sympatrically on leaf litter and correspond to dominant species in the Atlantic Rainforest of northeastern Brazil [Citation22]. Ananteris mauryi is a small species (adult size, 13.6–24.1 mm) and an active hunter, roaming mainly through the bottom layers of leaf litter. Tityus pusillus (adult size, 30–35 mm) is a sedentary scorpion hunting in the upper layers of leaf litter, waiting for prey in an ambush posture [Citation7,Citation22]. Despite this microhabitat stratification, T. pusillus was reported preying A. mauryi individuals [Citation19]. In addition, A. mauryi individuals are able to detect chemical cues of T. pusillus in substrate adopting a more cautious behavior [Citation23]. Therefore, considering that small scorpions avoid areas with the presence of larger species, in the present study, we investigate A. mauryi foraging activity and microhabitat choice in sites with different T. pusillus density.
Methods
Study area
This study was conducted in five Atlantic Rainforest remnants (49.6–1,397.6 ha) located in Pernambuco State, northeastern Brazil (, ). All fragments were surrounded by sugarcane crops or human settlements. The sampled sites were composed of the evergreen Atlantic Rainforest remnants largely dominated by Lecythidaceae, Sapotaceae, Moraceae, and Fabaceae tree species [Citation24]. Average rainfall over the study period was 39.08 and 248 mm (September–February 2014) [Citation25].
Figure 1. Remnants of the Atlantic Rainforest sampled (solid circles) in Pernambuco state, northeast. 1) Água Preta, 2) Jaqueira, 3) Tamandaré, 4) Ipojuca, and 5) Moreno.
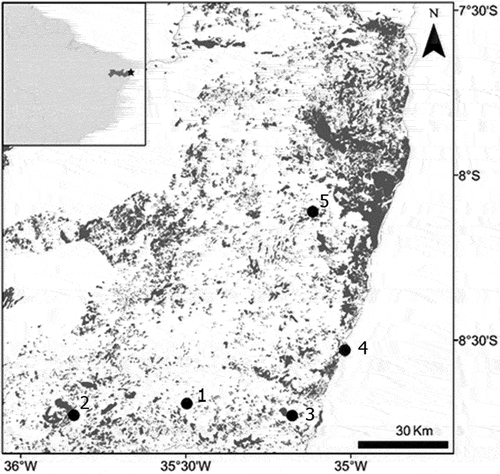
Table 1. Abundance of leaf litter-dwelling scorpions and species ratio (Ananteris mauryi:Tityus pusillus) in five Atlantic Rainforest remnants, Brazil.
Scorpion foraging activity and microhabitat choice
Scorpions were sampled during two nights (19:00–22:00) in each Atlantic Rainforest remnant. In each site, six quadrats (30 m × 10 m) were sampled per night for 1 h by a pair of collectors equipped with UV lamps. During the active search, the postures and microhabitat of each scorpion were recorded following the procedures described in previous studies [Citation7,Citation22]. For leaf litter stratification, we adopt the following approach: the top layer (2–3 cm depth) primarily consisted of intact leaves and fallen branches. The bottom layer (3–4 cm depth) consisted mostly small pieces of leaves and branches in different states of decomposition [Citation7,Citation22]. Voucher specimens were deposited in the Arachnological Collection of Universidade Federal de Pernambuco.
Results
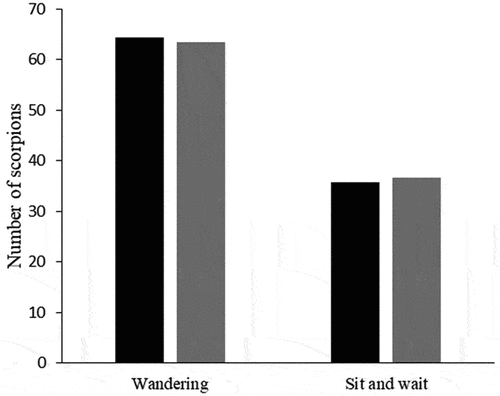
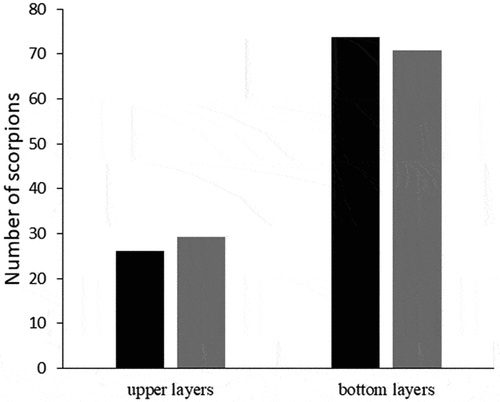
A total of 380 scorpions were collected being 280 T. pusillus and 81 A. mauryi. Most of the A. mauryi individuals (49.4%) were recorded in the Água Preta remnant (). The ratio A. mauryi/T. pusillus in this remnant was 20:1, while in other forest remnants, this ratio ranged between 0.07 and 0.10:1 (). Ananteris mauryi individuals were mostly (higher density: 70.8% and lower density: 74%) found in the bottom than top (higher density: 29.2% and lower density: 26%) layers of leaf litter independently of T. pusillus density (). Likewise, A. mauryi individuals were similarly found foraging on the bottom layers of leaf litter in remnants with higher and lower T. pusillus density ().
Discussion
In the present study, we analyzed the effects of predator density (T. pusillus) on foraging behavior and the choice of microhabitat of A. mauryi (prey) in fragments of the Brazilian Atlantic Rainforest. We found that A. mauryi foraging behavior and microhabitat selection were not influenced by T. pusillus density. This absence of a relationship may be motivated by predation pressure by other arthropods. In the Brazilian Atlantic Rainforest, different predatory arthropods (e.g. assassin bugs, spiders and ants) have been reported to feed on scorpions [Citation26–28]. Thus, colonizing the upper layers of the litter would increase the exposure of animals and the subsequent risk of predation. Another possible explanation may be related to the ecological requirements of A. mauryi. Small invertebrates avoid microhabitats under temperature and humidity conditions that cause water loss [Citation29]. Thus, small scorpions such as Ananteris spp. have a greater dependence on wet microhabitats with more stable microclimates, such as the bottom leaf litter layers [Citation22,Citation30,Citation31].
The stress caused by the presence of a potential predator can cause changes in the behavior of their prey [Citation2,Citation8,Citation32]. Previous studies have reported that arachnids can detect and change their behavior when exposed to chemical cues from their predators [Citation8,Citation33,Citation34]. Despite this, our results show that the relationship with T. pusillus density did not influence the foraging activity and microhabitat selection of A. mauryi in the Brazilian Atlantic Rainforest.
Acknowledgments
We are grateful to Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco-FACEPE for postdoctoral scholarship (BFP-0121-2.05/20). We also thank two anonymous referees for their valuable comments on the previous version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Werner EE, Anholt BR. Predator‐induced behavioral indirect effects: consequences to competitive interactions in anuran larvae. Ecology. 1996;77(1):157–169.
- Nelson EH, Matthews CE, Rosenheim JA. Predators reduce prey population growth by inducing changes in prey behavior. Ecology. 2004;85(7):1853–1858.
- Cresswell W. Non-lethal effects of predation in birds. Ibis. 2008;150(1):3–17.
- Brown JS, Laundré JW, Gurung M. The ecology of fear: optimal foraging, game theory, and trophic interactions. J Mammal. 1999;80(2):385–399.
- Křivan V, Schmitz OJ. Trait and density mediated indirect interactions in simple food webs. Oikos. 2004;107(2):239–250.
- Preisser EL, Bolnick DI, Hector A. The many faces of fear: comparing the pathways and impacts of nonconsumptive predator effects on prey populations. PLoS One. 2008;3(6):2465.
- Lira AFA, Souza AM, Filho ACS, et al. Spatio-temporal microhabitat use by two co-occurring species of scorpions in Atlantic rainforest in Brazil. Zoology. 2013;116(3):182.
- Dionisio-da-Silva W, Lira AFA, Albuquerque CMR. Prey-predator interactions between two intraguild predators modulate their behavioral decisions. Acta Ethol. 2019;22(3):195–201.
- Schmitz OJ. Effects of predator hunting mode on grassland ecosystem function. Science. 2008;319(5865):952–954.
- Holt RD, Polis GA. A theoretical framework for intraguild predation. Am Nat. 1997;149(4):745–764.
- Polis GA, Myers CA, Holt RD. The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu Rev Ecol Evol Syst. 1989;20:297–330.
- Polis GA, McCormick SJ. Patterns of resource use and age structure among species of desert scorpion. J Anim Ecol. 1986;55(1):59–73.
- Polis GA, McCormick SJ. Intraguild predation and competition among desert scorpions. Ecology. 1987;68(2):332–343.
- Kondoh M. Building trophic modules into a persistent food web. Pnas. 2008;105(43):16631–16635.
- Stouffer DB, Bascompte J. Understanding food‐web persistence from local to global scales. Ecol Lett. 2010;13(2):154–161.
- Ryan D, Cantrell RS. Avoidance behavior in intraguild predation communities: a cross-diffusion model. Discrete & Continuous Dynamical Systems – A. 2015;35(4):1641–1663.
- Brownell P, Polis GA. Scorpion biology and research. Oxford: Oxford University Press; 2001.
- Moreno-González JA, Hazzi NA. Intraguild predation case: Tityus forcipula Gervais, 1843 (Scorpiones, Buthidae) feeding on Chactas vanbenedeni Gervais, 1843 (Scorpiones, Chactidae) in Colombia. Rev Ibér Aracnol. 2012;20:117–120.
- Lira AFA, DeSouza AM, Albuquerque CMR. Report of intraguild predation and cannibalism in scorpions (Scorpiones: Buthidae) in the Brazilian Atlantic forest. North-Western J Zool. 2017;13(2):356–358.
- Urbani P, Ramos-Jiliberto R. Adaptive prey behavior and the dynamics of intraguild predation systems. Ecol Modell. 2010;221(22):2628–2633.
- Sánchez-Piñero F, Urbano-Tenorio F. Watch out for your neighbor: climbing onto shrubs is related to risk of cannibalism in the scorpion Buthus cf. Occitanus PLoS One. 2016;11(9):1–18.
- Lira AFA, DeSouza AM, Albuquerque. Environmental variation and seasonal changes as determinants of the spatial distribution of scorpions (Arachnida: Scorpiones) in neotropical forests. Can J Zool. 2018;96(9):963–972.
- Feitosa MLB, Dionisio-da-Silva W, Lira AFA, et al. Fear as an enemy? Behavioral changes of Ananteris mauryi (Scorpiones: Buthidae) triggered by chemical cues from an intraguild predator. Can J Zool. 2022;100(8):488–493.
- Lima DA. Estudos Fitogeográficos de Pernambuco. An Acad Pernamb Ciênc Agro. 2007;4:243–274.
- APAC. Agência Pernambucana de Águas e Clima. Last accessed: May 2017. http://www.apac.pe.gov.br/.
- Lira AFA, Foerster SAI, Silva-Filho AAC. Reports of scorpion predation by spiders in the Brazilian Atlantic forest and Caatinga (Arachnida: Scorpiones, Araneae). Rev Ibér Aracnol. 2016;28:87–89.
- Lira AFA, Araújo VLN, Albuquerque CMR. Predation of a scorpion (Scorpiones: Buthidae) by an assassin bug (Heteroptera: Reduviidae) in the Brazilian Atlantic forest. Turk J Zool. 2016;40:294–296.
- Dionisio-da-Silva W, Lira AFA. Record of Ananteris mauryi (Scorpiones: Buthidae) preyed upon by Ectatomma planidens (Hymenoptera: Formicidae) in the Brazilian Atlantic rainforest. Entomol News. 2019;128(5):497–503.
- Cloudsley-Thompson CL. Microclimates and the distribution of terrestrial arthropods. Annu Rev Entomol. 1962;7(1):199–222.
- Lourenço WR. Humiculous scorpions: on the genera Ananteris Thorell, 1891 and microananteris Lourenço, 2004 (Scorpiones: Buthidae), with the description of a new species from French Guiana. C R Biol. 2012;335(8):555–561.
- Lourenço WR. Comments on the Ananterinae Pocock, 1900 (Scorpiones: Buthidae) and description of a new remarkable species of Ananteris from Peru. C R Biol. 2015;338(2):134–139.
- Sheriff MJ, Peacor SD, Hawlena D, et al. Non‐consumptive predator effects on prey population size: a dearth of evidence. J Anim Ecol. 2020;89(6):1302–1316.
- Okuyama T. The role of antipredator behavior in an experimental community of jumping spiders with intraguild predation. Pop Ecol. 2018;44(2):121–125.
- Nisani Z, Honaker A, Jenne V, et al. Evidence of airborne chemoreception in the scorpion Paruroctonus marksi (Scorpiones: Vaejovidae). J Arachnol. 2018;46(1):40–44.