ABSTRACT
The movements and habitat use of three species of Sigmodontine rodents (Akodon mollis, Phyllotis haggard and Thomasomys paramorum) from the high Andes of Ecuador were evaluated using the spool-and-line technique in four transects. The floristic composition was analyzed at sampling sites to explain how these three species of Sigmodontine rodents use the vegetation as a habitat. Rodents were captured using live Sherman traps and equipped with a spool-and-line device. Each thread trail was traced, and their nests, feeders and shelters were recorded using GPS receivers. Also, the construction materials, nest morphology and shelters used during foraging, and the food resources were described. These three rodent species overlap in their distribution, but they present certain preferences for different habitats such as grasslands, open areas with cushions and shrubs, respectively. This study is an approach to the natural history of these Sigmodontine rodents in high mountain elevations.
Introduction
Several studies in the Neotropics on how cricetid rodents use and interact with their habitat have been carried out with the capture-recapture methodology [Citation1–4]. This methodology allows to know the state of rodent populations in various types of ecosystems, and their function, such as: seed dispersal [Citation1,Citation5], control of plant demography [Citation6] or insect population control [Citation7]. Other methods such as radio-telemetry [e.g. Citation2,Citation8], tracking powder [e.g. Citation9], and the spool-and-line technique [Citation10,Citation11] have been used less frequently [Citation12]. The spool-and-line technique is a tool that provides information about the natural history of rodents and other small and medium-sized mammals starting on their activity areas without the need for recapture [Citation2,Citation12–16].
In South America, the spool-and-line technique has been used in some rodent species and different habitats, such as Cerrado and semi-deciduous forests of Brazil, Andean montane forests, and others [Citation3,Citation13,Citation15–18] to increase data on natural history and habitat use of the species studied. However, the ecological interactions between rodents and their environment in ecosystems above 4000 m, as in the case of the Andean páramo, remain unknown, despite the high levels of diversity and endemism that these ecosystems harbor. In the páramos, the events of geographic isolation, diversification, and extreme environmental conditions resulting from altitude, low temperatures, wind, low atmospheric pressure, and high solar radiation, generate different climatic scenarios throughout the day. These forced species to develop ecological, physiological, physical, and mechanical adaptations very different from those observed in other Neotropical ecosystems [Citation19,Citation20], leading to high biodiversity.
For example, in Ecuadorian paramos, 72 species of mammals and 1500 species of plants have been recorded, of which 18 (39.1 %) and 270 (18 %), respectively, are endemic to the region [Citation20,Citation21]. In Ecuadorian paramos, the richness of rodents amounts to 29 species [Citation22], equivalent to 40% of the mammalian diversity recorded for this bio43me.
The present study focuses on the use and, a brief approach to, the habitat preference of three species of Sigmodontine rodents from the páramo of the Antisana volcano, using the spool-and-line technique, thus providing relevant information about their natural history.
Methods
Study area
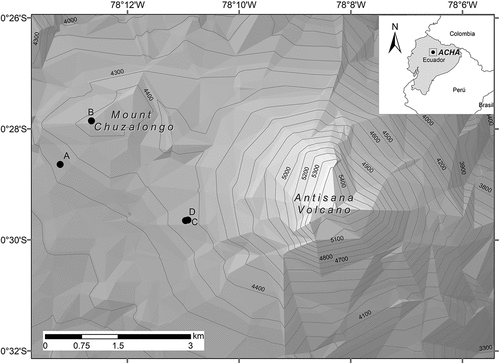
The study was carried out between August and October 2018 within the “Área de Conservación Hídrica Antisana (ACHA)” in the Northern Andes, Napo province of Ecuador () on the western slopes of the Antisana volcano between 4200 and 4500 m. The climate on the western slopes of the volcano is cold, with an average temperature of 3° C. The area has two rainy seasons between April to June and October to November, with an annual average precipitation of 1093 mm and receives up to 35% of solar radiation from August to September [Citation23].
Figure 1. Location of the four transects within the Área de Conservación Hídrica Antisana (ACHA), western slopes of the Antisana volcano, Napo Province, Ecuador.
The study area is located within a private conservation area dedicated to protecting the hydrographic basins of the rivers Pita, Quijos and Antisana, and to the natural regeneration of the páramo [Citation24]; it consists of about 20,000 hectares and is adjacent to the “Reserva Ecológica Antisana” (REA). Its vegetation is dominated by grasslands, wetlands and high Andean forests and shrublands [Citation25]. According to the Sistema de Clasificación de Ecosistemas de Continental Ecuador [Citation26], the chosen sampling sites were distributed among the ecosystems of Herbazal del Páramo, Herbazal ultrahúmedo subnival del páramo y Herbazal y Arbustal siempreverde subnival del Páramo.
Sampling design
In August 2018, four 200 m x 20 m transects were established in grassland, shrubland, and cushion plant areas (). To determine the type of habitat, the flora was characterized in each transect using a 1 m2 grid divided into 20 × 20 cm quadrants [Citation27]. The coverage and growth forms of each registered species were determined [Citation27,Citation28]. Plant specimens from each transect were collected and later transferred for identification to herbarium QCA of the Pontificia Universidad Católica del Ecuador (Appendix A).
Table 1. Information about transects. A. Herbazal del páramo, B. Herbazal ultrahúmedo subnival del páramo, C. Arbustal siempreverde subnival del páramo. D. Herbazal del páramo.
Capture and management of rodents
Rodent capture was carried out in two field trips from September to October 2018, eight days each, for a total of 16 days of effective sampling. Sixty Sherman live traps were placed in 20 stations along each transect (three traps per station) and were activated for 24 hours, baited with a combination of peanut paste, banana, oatmeal, and vanilla essence. The geographic coordinates of the capture sites were marked, and the captured individuals were marked with temporary dyes on the legs and tails to avoid replications [Citation15,Citation29]. The animals were handled according to the guidelines of the Sección de Mastozoología del Museo de Zoología de la Pontificia Universidad Católica del Ecuador (QCAZ) [Citation30] and the American Society of Mammalogists [Citation31].
Some rodent specimens were collected, and handled according to defined protocols [Citation30], and deposited at QCAZ to confirm the field taxonomic identifications using specialized literature [Citation32–34] and direct comparisons with museum material (Appendix B).
Movement
To characterize the movement of the rodents, the captured individuals were equipped with a spool and line device, according to the methods proposed in the literature [Citation10,Citation11,Citation35]; the spool was adhered to the dorsal coat with Uro-Bond IV glue [Citation17]. Rodents were released at the same capture site and their movement was monitored the next day as suggested in [Citation15,Citation35]. The threads were recorded on GPS receivers, emulating the individual’s movement; additionally, points of nests, feeders, and shelters were recorded and described [Citation3,Citation18,Citation36]. In the case of vertical movement, the length of the thread was measured on the shrub vegetation. Based on the movement activity, the type of locomotion of the rodent species was also classified as terrestrial, climbing, or semi-fossorial; that is, if their routes were under or above the ground or the shrub vegetation, respectively [Citation37].
Description of sites of interest
Sites of interest were classified and recorded according to the following definition:
Nest = sites of habitual permanence, used for shelter from climate, rest, give birth to their young, and give parental care [Citation8,Citation38]; plant fibers are intentionally taken for their construction [Citation17,Citation18,Citation39].
Shelter = cavity naturally formed or built by some other animal, it is a temporary dwelling place during the day that can be used by a passing animal if needed [Citation8,Citation10,Citation40].
Feeder = a site where an unusual abundance of food and feces remains is found [Citation41].
Each nest, feeder, and shelter were measured and characterized based on their shape and size. In the case of nests, the materials used for their construction were described [Citation2,Citation8,Citation17,Citation42]. Photographs were taken, and food and feces samples were collected in feeders and shelters. The number of sites of interest found in each habitat and for each rodent species was also counted.
Data analysis
Floristic characterization
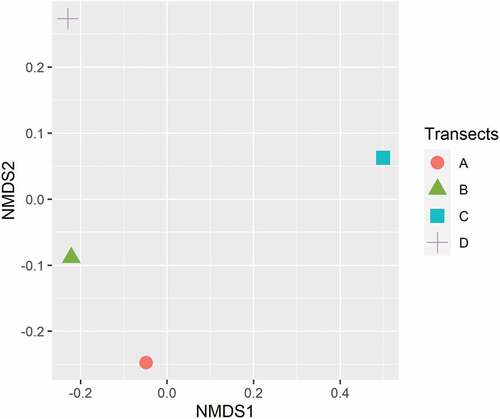
Vegetation composition was compared between transects. The incidence of species and their percentage of coverage was analyzed through a non-metric multidimensional scaling analysis (NMDS) [Citation43,Citation44].
Use of habitat
Rodent’s movement information was processed in the ArcGIS 10.6.1 package [Citation45]. Routes and points of interest were drawn in maps to generate quantitative and visually descriptive information on each rodent movement.
Habitat preference
Habitat preference analysis was carried out in the HaviStat © V2.3 program developed by Montenegro and Acosta [Citation46]. Bonferroni index with confidence intervals was used to determine the preference or use of habitat [Citation47], the variables were the type of habitat (bush, grassland, and cushions) and the number of sites of interest (nests, feeders, and shelters) recorded in each habitat and for each rodent species.
A G-test goodness-of-fit analysis was also performed to determine if habitat preferences were significant, using the same habitat preference variables. Additionally, the Cherry test was used to evaluate the sample size for habitat preference based on two confidence intervals [Citation47]. If the sample size passed one of the two tests when the error did not exceed 5%, the results would be reliable to infer preference.
Results
Floristic characterization
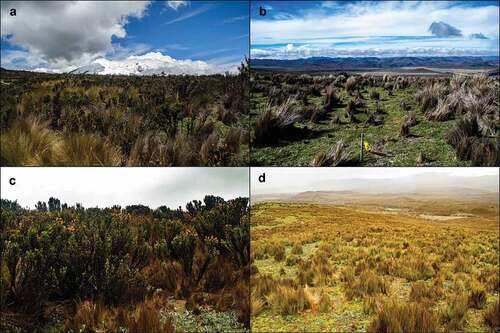
Eight growth forms and 41 plant species belonging to 18 families were recorded, of which the most representative families were Asteraceae and Poaceae. Site A was composed mainly of Chuquiraga jussieui shrubs and Calamagrostis intermedia tuft, which was classified as a mixed shrub and grass habitat. Site B was composed mostly of cushions of Plantago rigida and with a small tuft of Festuca chimborazensis, it was classified as an open cushion habitat. Site C was composed mainly of shrubs of C. jussieui and was classified as a shrubby habitat. Lastly, Site D was largely composed of tuft of C. intermedia, which was classified as a grassland habitat (Appendix A; ).
Figure 2. Four sampling sites with different vegetation: A. Herbazal del páramo, B. Herbazal ultrahúmedo subnival del páramo, C. Arbustal siempreverde subnival del páramo. D. Herbazal del páramo.
The sampling sites showed significant differences concerning the vegetation covers according to the NMDS analysis (S = 0.11; P = 0.001; ).
Rodent capture
After 16 days of sampling, with a cumulative effort of 3,840 traps/night, 59 individuals were captured (Appendix C), belonging to three species of rodents (Cricetidae: Sigmodontinae): the Soft-furred Grass Mouse, Akodon mollis Thomas, 1854 (n = 35, 59% of the total) was recorded in four sites; the Páramo Andean Mouse, Thomasomys paramorum Thomas, 1898 (n = 17, 29%) was recorded at three sites; and the Haggard’s Leaf-eared Mouse, Phyllotis haggardi Thomas, 1898 (n = 7, 12%) was present in two sampling sites ().
Table 2. Summary of data from rodent’s species.
Movement
Forty-three individuals (27 A. mollis, 11 T. paramorum, and 5 P. haggardi) were subjected to the spool-and-line technique and followed, ten of them were recaptured and released without applying the spool again ().
For A. mollis, the lengths of the movement recorded were between 32.86 to 158.52 m. Its activities and tracks were observed mostly above ground but also using galleries under the ground. Its locomotion was classified as terrestrial-semi-fossorial. For T. paramorum the lengths of its movements recorded were between 44.10 to 174.21 m. Its activities and tracks were observed more frequently on the ground and shrubs. This locomotion was classified as a terrestrial climber. For P. haggardi the lengths of their movement recorded were between 31.60 to 77.28 m. Its activities and tracks were all observed on the ground in open areas of vegetation. This locomotion was classified as terrestrial of open areas.
Description of sites of interest
Akodon mollis. – (males = 13; females = 14; between adults and subadults). From the tracks, a total of eight nests, seven shelters, and 15 feeders were recorded in the four sampling sites ().
Nests: A. mollis nests did not have a defined shape, they acquired the shape of the structure occupied. They have one to four entrances arranged in various directions, connected by internal tunnels. The entrances had diameters between 4–6 cm. The dimensions of the nest cavity were: 10–40 cm deep by 11–40 cm wide and 6–9 cm high. They were found on decayed A. pedunculata cushions, along with or under C. jussieui shrubs, or tuft of C. intermedia ()). In the internal chamber of the nests plant fibers of C. intermedia and bryophytes were found. Nests were apparently used to spend the night or rest. No food remains, or feces were found inside nests ()).
Figure 4. Akodon mollis in Ecuadorean páramo. A. Nest under Azorella pedunculata. B. Internal content of nests, vegetation peat of bryophytes, bark, and vegetable fibers is observed. C. Shelter between tuft of Calamagrostis intermedia. D. Remains of melolontin beetles found in shelters during the trail. E. Remains of Chuquiraga jussieui found under shrubs. F. Feeder type under C. jussieui shrub.

Shelters: These do not have definite shapes and are characterized by being small areas located under bushes of C. jussieui, Loricaria thuyoides, or under C. intermedia ()). Their dimensions were 17–23 cm long by 12–15 cm wide. They were found covered by bryophytes, litter and lichens. Rodent and Andean rabbit (Sylvilagus andinus) feces were found inside the shelters, as well as small plant scraps of C. jussieui, and animal remains of melolontin beetles (Scarabaeidae: Melolonthinae; )).
Feeders: These do not have defined shapes and are characterized by being mostly under the shade of C. jussieui shrubs that usually occupy an area of 1 m2. The size of the feeder area was 20–24 cm long by 11–15 cm wide. The feeders do not have entrances like the nests, but the grassland or the roots of shrubs form cavities that are the main shelter sites while the rodents feed on vegetation ().
Thomasomys paramorum. – (males = 8; females = 3; between adults and sub-adults). From the tracks, a total of five nests, three shelters, and 12 feeders were recorded in the three sampling sites ().
Nests: Nests of T. paramorum did not have a defined shape, they acquired the shape of the structure they occupied, and have a single entrance with a diameter of 6.0–6.2 cm. The nest dimensions were 20–42 cm deep by 17–40 cm wide and 7–9 cm high. They were found on decayed Azorella pedunculata cushions ()), along with C. jussieui shrubs. Inside the nests, apparently used to spend the night or rest ()), plant fibers of C. intermedia, bryophytes and feces were found. The bones and tail of an unidentified rodent were found in the nest of C3 ()).
Figure 5. Sites of interes registered for Thomasomys paramorum in Ecuadorean páramo. A. Nest under Azorella pedunculata. B. Internal content of nests, peat of vegetation of bark, and vegetal fibers is observed. C. Unidentified rodent bone found in nest. D. Shelter among tuft of Calamagrostis intermedia feces are observed. E. Remains of melolontin beetles found in shelters during the trail. F. Feeder type under C. jussieui shrub. G. Flowers of C. jussieui gnawed from a live plant.

Shelters: These do not have definite shapes; they are characterized by being under tuft of C. intermedia and can also be found under A. pedunculata cushions. Unlike the nests, inside the shelters, no plant remains were found to rest upon. They had approximate dimensions of 20–23 cm long by 13.0 cm wide. The floor was covered by bryophytes and lichens. Inside, rodent and S. andinus feces and small food remain of melolontin beetles were found ().
Feeders: These were mostly found under shrubs of C. jussieui ()) and to a lesser extent under tuft of C. intermedia. The description is similar to the feeders of A. mollis. Feeding trails were also found in the upper part of the bushes ()).
Phyllotis haggardi. – (males = 3; females = 2; between adults and sub-adults). From the tracks, one nest, three shelters, and a single feeder were recorded ()
Nests: The only nest recorded for P. haggardi was used by a male (B4; Table 4). The nest was round, 50.0 cm deep by 40.0 cm wide and 7.6 cm high and had a single entrance of 7.2 cm in diameter ()). It was located under a living cushion of Plantago rigida, and above, there was a Valeriana microphylla bush (). Fibers of C. intermedia were found inside the nest. It apparently was used to spend the night or rest ()).
Figure 6. Sites of interest registered for Phyllotis haggard in Ecuadorean páramo. A. Nest under Plantago rigida. B. Entrance to nest in a cushion of P. rigida. C. Nest under Valeriana microphylla. D. Internal content of nests, peat of vegetation of bark, and vegetal fibers is observed. E. Shelter observed under tuft of Calamagrostis intermedia, the floor is covered with bryophytes and feces of Sylvilagus andinus.
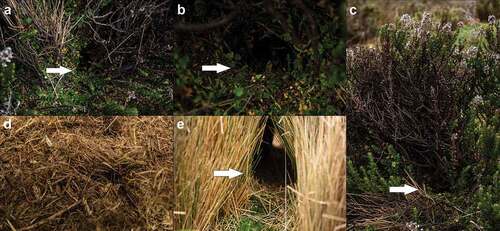
Shelters: These did not have definite shapes. They were found under tuft of C. intermedia, with average measurements of 21.3 cm long by 14.6 cm wide. Rodent and S. andinus feces were found inside, and the ground was covered by lichens and bryophytes ()).
Feeders: A single trough was recorded under tuft of C. intermedia, its dimensions were 23.0 cm long by 11.0 cm wide, with remains of Phlegmariurus crassus var. manus-diaboli inside.
Habitat use
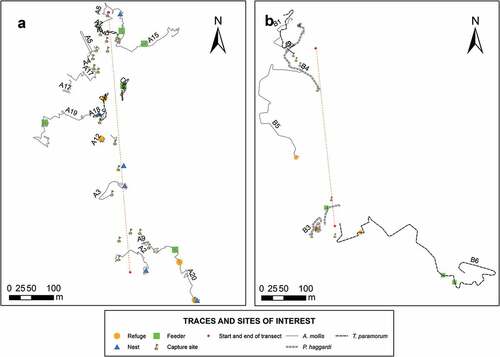
Movement maps showed overlapping life ranges of rodents, but no clear movement patterns were defined by the rodents studied. However, the use of nests, feeders, and shelters showed a pattern ( 7 ).
Figure 7. Movement map of individuals in the four sampling sites. Lines of different colors represent the movements of each cricetid rodent. Nests, feeders, and shelters are represented in figures. There is no observable movement pattern. The figure shows sampling sites A and B. The detailed information on the individuals is found in Appendix C.
Habitat preference
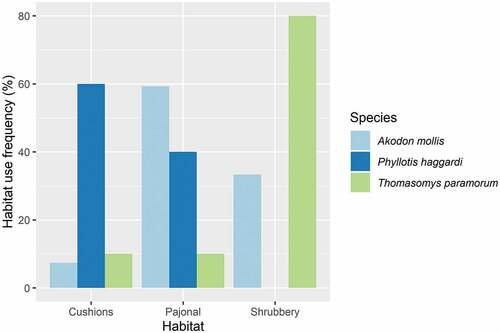
The analysis of habitat preference based on the Bonferroni index [46]) (8), shows that A. mollis occurs preferentially in grassland habitat 59%, followed by shrub habitat (33%), and lastly cushion plant habitat (7%). The G-test goodness-of-fit analysis shows highly significant values (p = 0.001) for the habitat preference of this species. Likewise, in the preference analysis, T. paramorum uses primarily shrub habitat (80%), followed by grassland habitat (10%), and lastly cushion habitat (10%). The goodness of fit analysis shows significant values (p = 0.035). Finally, P. haggardi has a high occurrence in the cushion plant habitat (60%) and slightly lower occurrence in grassland (40%); while actively avoiding bushes. On the other hand, the Cherry analysis for the evaluation of sample size on the habitat preference showed that only A. mollis meets a reliable sample size to assert a preference (Appendix D).
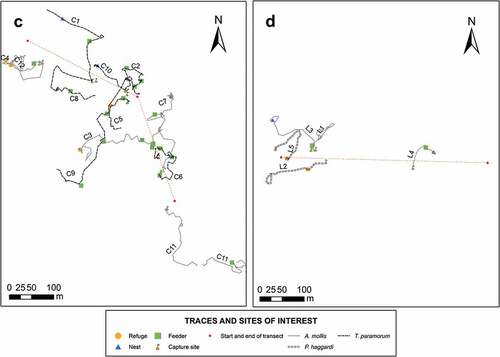
Figure 8. Movement map of individuals in the four sampling sites. Lines of different colors represent the movements of each cricetid rodent. Nests, feeders, and shelters are represented in figures. There is no observable movement pattern. The figure shows sampling sites C and D. The detailed information on the individuals is found in Appendix C.
Discussion
The movement of rodents determined from the spool-and-line technique reveals details about habitat use and preference, the first records for nests, feeders, and shelters, and the movement of A. mollis, T. paramorum, and P. haggardi in a high Andean ecosystem. The movement pattern of these rodent species is also suggested. These results will be discussed based on the different morphological and behavioral adaptations of each cricetid rodent.
Habitat use
Information on movement can be difficult to process due to the fall of the spool or its detachment by some of the rodents [Citation12,Citation14]. Nevertheless, the present study shows differences in the length of the movement between species, where T. paramorum presented the longest routes followed by A. mollis, and P. haggardi with the shortest routes (). Although no movement pattern was observed (), the movement of each species was related to the structure and same plant composition of the habitat [Citation2]. It is remarkable that the lengths of movements were different between sexes (). Males of the three species had the longest routes, perhaps because their life ranges are more extensive compared to females, whose life ranges would be related mainly to parental care, while males, as they present a larger body size, would need a larger foraging area to reach their energy requirements. Males can have several shelters and have more than one sexual partner [Citation8,Citation48].
The results also show that the three rodent species use common sites such as nests, feeders, and shelters. These sites would be chosen or built based on the availability of resources and floristic and structural requirements of each species [Citation2].
The nests of the three rodent species were found under cushions, both living and decomposing, at the four sampling sites (), 5(a), and 6(a)). Rodents would take advantage of the availability and structure of the cushions to build their nests. In addition, other studies [Citation49,Citation50] have shown that the internal temperature within the cushions is higher and more stable compared to ambient temperature, which would make them suitable for use as shelters, since, in addition to reducing the risks of predation, there would be a thermal advantage in the face of external climatic conditions [Citation2,Citation8,Citation18].
Another similarity between the three rodent species was the type of plant material found inside the cavities of nests. All three species used plant fibers and bryophytes as a “mattress” to spend the night or rest, similar to what has been observed in other studies [Citation8–37,Citation39,] (), 5(b), and 6(d)). This may confer an energy advantage by reducing the metabolic costs of thermoregulation [Citation39,Citation51], and avoid resting on the ground.
The difference between species lies in the number of entrances that nests have. In the case of A. mollis, having up to four interconnected entrances may be related to the semi-fossorial locomotion of the species, which would give it an escape advantage against predators in the area. In contrast, the nests of T. paramorum have only one entrance, a characteristic that does not agree with the descriptions in literature [Citation17], where nests had two entrances located in a north-south direction in an Andean forest at 3620 m. This could be a differential behavior of the species above 4000 m [Citation39,Citation51].
Another difference between species was related to the cleanliness of nests. In the nests of A. mollis, and the only registered nest of P. haggardi, no food remains or feces were found, which would prevent the proliferation of parasites or diseases, while in the nests of T. paramorum, food scraps and feces were found, including skeletal remains of an unknown rodent ()). Based on the evidence found, it might be possible that T. paramorum is an opportunistic rodent that does not expend energy in the construction of a nest, but instead takes advantage of the availability of shelters abandoned by other species, so the remains could belong to the previous occupant of the nest.
As for the feeders, both A. mollis and T. paramorum used shrubs of C. jussieui as foraging sites, where they possibly feed on the seeds of the shrub ()). The difference with T. paramorum was that it also feeds on Chuquiraga seeds in the upper part of the bushes ()), being the only rodent that showed vertical movement. The rodents might not only act as granivores and controllers of the Chuquiraga demography, but they could also act as accidental dispersers of its seeds since remains of the flowers were found under tuft of C. intermedia that were far from the producing shrubs. Chuquiraga jussieui produces flowers throughout the year, so its seeds could be a stable food source for rodents and other animals.
In the trough recorded for P. haggardi no abundant remains of a specific food were observed, although remains of the lycophyte Phlegmariurus crassus var. manus-diaboli and gnawed plants were found along the way [Citation52]. Until now, information on the feeding of this species and foraging mode was unknown [Citation53,Citation54], so this would be the first record of these behaviors. Few mammals consume lycophytes because they contain high levels of alkaloid compounds such as licodyn, lycopodin, huperzine A, B, and fawcetidine [Citation55]; alkaloids generate acute physiological reactions in animals [Citation56]. However, though alkaloids found in lycophytes are recognized as moderately toxic, it seems that there are no records of poisoning in animals [Citation57] due to the unappetizing nature of these plants.
On the other hand, under the tuft of C. intermedia, natural shelters are formed for rodents and other mammals such as S. andinus, which would use the vegetation to avoid exposure to the wind and rain [Citation58]. At these sites, remains of coleopterans of the Melolonthinae subfamily (family Scarabaeidae) were found. The beetles could provide a protein and lipid contribution to the diet of rodents [Citation1]. Beetles are considered a type of temporary feeding used in September and October when there are many adults present in the breeding season. This resource would not be consumed in abundance throughout the year [Citation7,Citation59].
Habitat preference
The habitat preference of the rodent species presented in this study is explained from the morphological and behavioral characteristics of the species, including the type of locomotion. In that sense, the route of A. mollis showed that the species uses the lower strata of the grassland or shrubland in the four sampling sites as its most common habitat, and its morphological characteristics such as the size of ears, short tails, eyeball diameter, and length of the claw support these habitat preferences (). Another notable adaptation is the ability to dig, providing them with fossorial capacity [Citation60]. Interconnected tunnels were found in most of its nests reaching depths greater than 40 cm, this characteristic would support the classification of this species as semi-fossorial because A. mollis was captured on top of the strata.
In contrast, T. paramorum was found mostly in places where shrubs were abundant, with a preference for areas dominated by C. jussieui, although it was also captured among shrubs of Loricaria thuyoides at Site B. The paths of these rodents were tracked mostly in the bush, avoiding open areas with cushions and grassland (; Appendix D). This high occurrence in the shrub areas allows us to conclude that T. paramorum, unlike A. mollis, is a species with climbing abilities, that frequents the highest strata of the shrubs, even reaching up to one meter high in shrubs of C. jussieui, as observed in this study. Morphological characteristics of T. paramorum are typical of certain rodents classified as bush climbers. Among these characteristic are short hind legs, a fifth semi-opposable digit, protruding and small separate toe pads that stand out. These are characteristics that give the rodent flexibility in its locomotion, ability to grip branches, a high capacity to reduce the impact created for falls [Citation61]; and a long tail would provide an advantage when moving between branches of bushes, providing balance and support [Citation61].
P. haggardi, on the other hand, was found in open areas and among the grasslands where no shrub vegetation was recorded. P. haggardi has long, slender hind legs, a medium-sized tail, and somewhat reduced clawed toes compared to the other two species in the study. These morphological characteristics allow it to move quickly between these areas [Citation60]. Other characteristics that stand out are their large eyes and ears specially adapted to nightlife. Their nocturnal habits would explain why P. haggardi prefered open areas, since during the night the number of aerial predators is reduced in the paramo [Citation62].
This open area habitat preference was not known for P. haggardi, as very little is known about its natural history [Citation53,Citation54]; although in the study [Citation63] individuals of P. haggardi were captured in an open wasteland area. Also, in northern Peru, another species, Phyllotis pearsoni, has a similar habitat preference in the Puna region where vegetation is dominated by herbaceous grassland and open areas [Citation64], similar to Sites B and D of this study. Therefore, it is suggested that these habitat preferences occur at the level of the Phyllotis genus in Ecuador, although the phylogenetic relationships between P. pearsoni and P. haggardi are still poorly known [Citation65].
The spool-and-line technique used in this study has allowed an approximation of the use and habitat preferences of rodent species, that demonstrates how related species can coexist in the same habitat if the resources are used differentially to avoid direct competition. There are many open questions regarding the ecology of these cricetid rodents within the high Andean ecosystems. However, this study is the first detailed approach to habitat use and preference. It is important to carry out similar studies in other areas and with other cricetid species to increase knowledge of their ecology and ethology.
Author contributions
SFB and AV conceived and designed the study. AV and RZC analyzed the data. RZC identified botanical specimens in situ and in the QCA Herbarium. AV and RZC wrote the first draft of the manuscript. RZC, JB and SFB reviewed and improved the manuscript.
Acknowledgments
The fieldwork was supported by the Museo de Zoología de la Pontificia Universidad Católica del Ecuador (QCAZ). Research and collection permits were provided by the Ministerio del Ambiente (011-2018-IC-FAU-DNB/MA and 037-2019-IC-FLO-DNB/MA). We thank Nicolás Tinoco who support us on fieldwork. We are also thankful to Florencio Maza who help us with beetle fragment identification, and to Domenica Naranjo, Lou Jost and María Eugenia Ordoñez for reviewing and improving the English version of this paper. We thank Ulyses Pardiñas and the reviewers in this work for their critical and helpful comments. Finally, we are thankful for the Fondo para la Protección del Agua (FONAG) and their constant effort in conservating Ecuadorian páramos, irreplaceable sources of water.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Montenegro-Díaz O, López-Arévalo H, Cadena A. Aspectos ecológicos del roedor arborícola Rhipidomys latimanus Tomes, 1860, (Rodentia: cricetidae) en el oriente de Cundinamarca, Colombia. Caldasia 1991;16(79):565–572.
- Wells K, Lakim MB, Pfeiffer M. Nest sites of rodents and treeshrews in Borneo. Ecotropica 2006;12:141–149.
- Prevedello JA, Rodrigues RG, de Monteiro-Filho ELA. Habitat selection by two species of small mammals in the Atlantic Forest, Brazil: comparing results from live trapping and spool-and-line tracking. Mamm Biol. 2010;75(2):106–114.
- Gomez D, Sommaro L, Steinmann A, et al. Movement distances of two species of sympatric rodents in linear habitats of Central Argentine agro-ecosystems. Mamm Biol. 2011;76(1):58–63.
- Sahley CT, Cervantes K, Pacheco V, et al. Diet of a sigmodontine rodent assemblage in a Peruvian montane forest. J Mammal. 2015;96(5):1071–1080.
- Rumiz DI. Roles ecológicos de los mamíferos medianos y grandes. Distrib Ecol Y Conserv Los Mamíferos Median Y Gd Boliv. 2010;2:53–73.
- Lopez‐Arevalo H, Montenegro‐Diaz O, Cadena A. Ecología de los pequeños mamíferos de la Reserva Biológica Carpanta, en la Cordillera Oriental colombiana. Stud Neotrop Fauna Environ. 1993;28(4):193–210.
- Moraes Junior EA, Chiarello AG. Sleeping sites of woolly mouse opossum Micoureus demerarae (Thomas)(Didelphimorphia, Didelphidae) in the Atlantic forest of south-eastern Brazil. Rev Bras Zool. 2005;22(4):839–843.
- Lemen CA, Freeman PW. Tracking mammals with fluorescent pigments: a new technique. J Mammal. 1985;66(1):134–136.
- Miles MA, De Souza AA, Póvoa MM. Mammal tracking and nest location in Brazilian forest with an improved spool‐and‐line device. J Zool. 1981;195(3):331–347.
- Boonstra R, Craine ITM. Natal nest location and small mammal tracking with a spool and line technique. Can J Zool. 1986;64(4):1034–1036.
- Mendonca AF, Bocchiglieri A, Vieira MV. Spool-and-line in a backpack: a new technique for studying movement of small mammals. Mamm. 2010;74(2):209–211 .
- Vieira EM, Iob G, Briani DC, et al. Microhabitat selection and daily movements of two rodents (Necromys lasiurus and Oryzomys scotti) in Brazilian Cerrado, as revealed by a spool-and-line device. Mamm Biol. 2005;70(6):359–365.
- Steinwald MC, Swanson BJ, Waser PM. Effects of spool-and-line tracking on small desert mammals. Southwest Nat. 2006;51(1):71–78.
- Almeida AJ, Freitas MMF, Talamoni SA. Use of space by the Neotropical caviomorph rodent Thrichomys apereoides (Rodentia: echimyidae). Zool. 2013;30(1):35–42.
- Noss AJ. Seguimiento del corechi (Tolypeutes matacus) por medio de carreteles de hilo en el Chaco boliviano. Edentata. 2013;14(1):15–22.
- Brito J, Teska WR, Ojala-Barbour R. Descripción del nido de dos especies de Thomasomys (Cricetidae) en un bosque alto-andino en Ecuador. Therya. 2012;3(2):263–268.
- Machado AF, Marks CF, Peres B, et al. Movement and use of environmental structures, climbing supports and shelters by Akodon montensis (Sigmodontinae, Rodentia) in the Atlantic forest of southern Brazil. Mammalia. 2019;84(1):107–113.
- Buytaert W, Célleri R, De Bièvre B, et al. Hidrología del páramo andino: propiedades, importancia y vulnerabilidad. Cuenca Recuper 2006. https://www.paramo.org/files/hidrologia_paramo.pdf
- León-Yánez S, Valencia R, Pitman N, et al. Libro rojo de las plantas endémicas del Ecuador. 2019.
- Tirira D, Brito MJ, Burneo S Mamíferos del Ecuador: lista actualizada de especies/Mammals of Ecuador: updated species check list (2020.1). 2020.
- Brito J, Camacho MA, Romero V, et al. Mamíferos del Ecuador. Museo de Zoología. Quito (Ec): Pontificia Universidad Católica del Ecuador; 2018.
- Sklenar P, Kucerova A, Mackova J, et al. Temporal variation of climate in the high elevation páramo of Antisana, Ecuador. Geogr Fis e Din Quat. 2015;38(1):67–78.
- FONAG. Fondo para la protección del agua. Available from: https://bit.ly/2RXqhOp
- Dinerstein E, Olson D, Joshi A, et al. An ecoregion-based approach to protecting half the terrestrial realm. Bioscience. 2017;67(6):534–545.
- Ministerio del Ambiente del Ecuador. Sistema de clasificación de los ecosistemas del Ecuador continental. Quito: Subsecretaría de Patrimonio Natural; 2012.
- Báez S, Cuesta F, Muriel P, et al. Monitoreo de biodiversidad, productividad y experimentación en ecosistemas herbáceos andinos. Protocolo 4-Versión 1. Quito EC: CONDESAN/Escuela de Ciencias Biológicas Biológicas-PUCE/IER-UNT; 2014.
- Sklenář P, Luteyn JL, Ulloa CU, et al. . Generic Flora Páramo Illus Guid Vasc Plants. Bronx (NY): New York Botanical Garden (US); 2005.
- Romero Almaraz ML. Mamíferos pequeños: manual de téchnicas de captura, preparación, preservación y estudio. CDMX: Universidad Nacional Autónoma de México; 2000.
- Camacho MA Registro de datos, preparación y preservación de los especímenes mastozoológicos: procesamientos para Colectas de Campo. Pontif Univ Católica del Ecuador Mus Zool sección Mastozoología Recuper. 2014. https://bitly/2CAGNBc
- Sikes RS. Mammalogists AC, of the American society of UC. 2016 guidelines of the American society of mammalogists for the use of wild mammals in research and education. J Mammal. 2016;97(3):663–688.
- Patton JL, Pardiñas UFJ, D’Elía G. Mammals of South America. Chicago: University of Chicago Press (US); 2015.
- D’Elía G, Pardiñas UFJ, Vorontsov TA. 1959. Mamm South Am. 2015;2:140–144.
- Voss RS. A new species of Thomasomys (Rodentia: muridae) from eastern Ecuador, with remarks on mammalian diversity and biogeography in the cordillera oriental. Am Museum Novit. 2003;2003(3421):1–47.
- Briani DC, Vieira EM, Vieira MV. Nests and nesting sites of Brazilian forest rodents (Nectomys squamipes and Oryzomys intermedius) as revealed by a spool-and-line device. Acta Theriol (Warsz). 2001;46(3):331–334.
- Kolbe JJ, Janzen FJ. Impact of nest‐site selection on nest success and nest temperature in natural and disturbed habitats. Ecology. 2002;83(1):269–281.
- Wells K, Pfeiffer M, Lakim MB, et al. Movement trajectories and habitat partitioning of small mammals in logged and unlogged rain forests on Borneo. J Anim Ecol. 2006;75(5):1212–1223.
- Ebensperger LA, Taborelli P, Giannoni SM, et al. Nest and space use in a highland population of the southern mountain cavy (Microcavia australis). J Mammal.2006;87(5):834–840.
- Rengifo EM, Aquino R. Descripción del nido de Scolomys melanops (Rodentia, Cricetidae) y su relación con Lepidocaryum tenue (Arecales, Arecaceae). Rev Peru Biol. 2012;19(2):213–216.
- Bonaventura S. Selección de hábitat por roedores en campos de cultivo. Un Estudio Experimental Physis. 1988;46:61–66.
- González Christen A Algunas interacciones entre Dioon edule (Zamiaceae) y Peromyscus mexicanus (Rodentia: cricetidae). 1990.
- Moura MC, Caparelli AC, Freitas SR, et al. Scale-dependent habitat selection in three didelphid marsupials using the spool-and-line technique in the Atlantic forest of Brazil. J Trop Ecol. 2005;21(3):337–342.
- Oksanen J, Kindt R, Legendre P, et al. The vegan package. Community Ecol Packag. 2007;10(631–637):719.
- Sánchez J. Introducción a la estadística no paramétrica y al análisis multivariado. Quito: Ecuador; 2004. p. 84–276.
- Esri R. ArcGIS desktop: release 10. CA: Environ Syst Res Institute; 2011.
- Montenegro J, Acosta A. Programa innovador para evaluar uso y preferencia de hábitat. Univ Sci. 2008;13(2):208–217.
- Cherry S. A comparison of confidence interval methods for habitat use-availability studies. J Wildl Manage. 1996; 60(3) :653–658.
- Cutrera AP, Antinuchi CD, Mora MS, et al. Home-range and activity patterns of the South American subterranean rodent Ctenomys talarum. J Mammal. 2006;87(6):1183–1191.
- Sklenár P, Ramsay PM Diversity of zonal páramo plant communities in Ecuador. Divers Distrib. 2001;7(3):113–124.
- Castro Mantilla MI Influencia de Azorella aretioides (Kunth) Willd. ex DC.(Apiaceae) y Xenophyllum rigidum (Kunth) VA Funk (Asteraceae) en el ensamblaje comunitario de plantas alto andinas, a lo largo de un gradiente altitudinal en la cara noroccidental del Volcán Antisana. 2019.
- Bonzinovic F, Merritt JF. Conducta, estructura y función de micromamíferos en ambientes estacionales: mecanismos compensatorios. Rev Chil Hist Nat. 1991;64:19–28.
- Zambrano-Cevallos R, Villarreal A, Yánez SL, et al. Unusual feeding record for a Neotropical rodent: a common Andean lycopfhyte.
- Tirira D.A field guide to the mammals of Ecuador [:] Including the Galapagos Islands and the Ecuadorian Antarctic Zone. Quito (EC): Asociación Ecuatoriana de Mastozoología y Editorial Murciélago Blanco; 2017.
- Wilson DE, Lacher TE, Mittermeier RA.Handbook of the mammals of the world: Vol. 7: Rodents II . Barcelona (ES): . Lynx Edicions; 2017.
- Aver W. Lycopodium alkaloids. In: Cordell G, editor. The alkaloids: chemistry and pharmacology. Chicago: Academic Press; 1994. p. 233–266.
- Manske RHF. The Lycopodium alkaloids In: The Alkaloids: Chemistry and Physiology. Cambridge (US): Elsevier Academic Press; 1955.p. 295–300.
- Kobayashi J, Morita H. The lycopodium alkaloids. In: the alkaloids: chemistry and biology. Elsevier; 2005;61:1–57.
- Camacho LF, Chávez P, Tirira DG. Elevation and wind exposure shape the habitat preferences of the Andean cottontail Sylvilagus andinus (Lagomorpha: leporidae). Mamm Biol. 2019;94(1):1–3.
- Carvajal V, Villamarín S, Ortega AM Escarabajos del Ecuador. Princ Géneros Inst Ciencias Biológicas Esc Politécnica Nac Ser Entomol Nro. 2011;1.
- Pearson OP. Taxonomy and natural history of some fossorial rodents of Patagonia, southern Argentina. J Zool. 1984;202(2):225–237.
- Cartmill M. Climbing. In: Hildebrand M, Bramble D, Liem K, et al., editors. Functional vertebrate morphology. Harvard: Belknap; 1985. p. 73–88.
- Cadena-Ortiz H, Solórzano MF, Noboa M, et al. Diet of the short-eared owl (Asio flammeus) in the Antisana highlands, Ecuador. Huit J Mex Ornithol. 2019;20(2): e–535.
- Barnett AA. Small mammals of the Cajas Plateau, southern Ecuador: ecology and natural history. Bull Florida Museum Nat Hist. 1999;42(4):161–217.
- Luebert F, Gajardo R. Vegetación alto andina de Parinacota (norte de Chile) y una sinopsis de la vegetación de la Puna meridional. Phytocoenologia. 2005;35(1):79–128.
- Pacheco V, Rengifo EM, Vivas D. A new species of Leaf-eared mouse, genus Phyllotis waterhouse, 1837 (Rodentia: cricetidae) from northern Peru. Therya. 2014;5(2):481–507.