ABSTRACT
The European Starling (Sturnus vulgaris) is a cavity-nesting bird with great invasive potential. As a result of human intervention, this bird is now distributed across all continents (except Antarctica) and its distribution range is increasing at an alarming rate. The European Starling was introduced to Argentina in 1983 and is currently distributed across almost the entire country. It is considered one of the hundred most damaging invasive species in the world and constitutes a serious competitive threat to native cavity-nesting birds. Interactions between European Starlings and cavity-nesting birds generally have negative consequences on native bird populations, although there are still few reports in the literature that account for the degree of damage. In this study, we report for the first time details of the harassment strategy and subsequent cavity usurpation by European Starlings on a breeding pair of Green-barred Woodpeckers (Colaptes melanochloros) in an urban area of central-eastern Argentina. Over one breeding season, the woodpeckers excavated seven cavities, none of which were successful. In six of these reproductive attempts (86%) we recorded interactions with European Starlings and in five (71%) the cavity was usurped. On three occasions we recorded a cooperative harassment strategy by a group of European Starlings causing the woodpeckers to abandon the cavity. Our report is especially relevant if we consider the invasive potential of the European Starling worldwide and the frequently limited cavity supply in bird breeding habitats. Therefore, we encourage governmental authorities and environmental NGOs to take measures to control the populations of this aggressive invasive species.
Introduction
Birds that nest in cavities constitute structured and complex communities that interact with each other mainly through competition for the use of these cavities [Citation1,Citation2]. Bird populations that form these communities are limited by the availability of natural cavities and/or suitable substrates for excavation [Citation3,Citation4], which are frequently reduced in human altered habitats [Citation5,Citation6]. In these habitats, primary forests have disappeared or been replaced by secondary forests [Citation1,Citation5,Citation7], which lack the large and mature trees key to cavity formation (either excavated or natural) [Citation8–11]. Primary cavity-nesters (cavity excavators) mainly depend on the availability of feasible wood to excavate [Citation7,Citation12], though they are not exempt of competition for existing cavities. Secondary cavity-nesters (non-excavator cavity users), however, mainly depend on cavities that already exist in the nesting habitat, so they must adapt to what they find and are more likely to compete with intra and interspecifics for the cavities that fit their needs [Citation8,Citation10]. Whatever the case, when cavity availability is very limited, breeding performance can be altered by the increased risk of cavity usurpation [Citation4,Citation13,Citation14].
The European Starling, Sturnus vulgaris, (Aves: Sturnidae) breeds in natural cavities, holes excavated by other birds or a variety of holes in human constructions [Citation15]. It was introduced to Argentina in 1983 [Citation16] and is currently widely distributed across the country [Citation17–21]. This species has been called one of the hundred devastating invasive species in the world [Citation22,Citation23], mainly because of its competition with native species [Citation24–26]. Across its range, the European Starling has been reported to compete with, harass, and usurp the cavities of woodpecker species, such as the Gila Woodpecker Melanerpes uropygialis [Citation27], Red-headed Woodpecker Melanerpes erythrocephalus [Citation28], White-fronted Woodpecker Melanerpes cactorum [Citation29], Northern Flicker Colaptes auratus [Citation30], Campo Flicker Colaptes campestris, and Green-barred Woodpecker Colaptes melanochloros [Citation26,Citation31,Citation32]. These studies agree that starlings are effective cavity usurpers, but no details have been specified about the strategy that allows this bird to successfully usurp cavities. In general, these interactions have negative population level consequences on primary cavity-nesters. Not only they increase nest abandonment rate and delay the beginning of reproduction [Citation26,Citation28,Citation33], most times they also provoke a forced energy investment of excavating a new cavity [Citation34].
In this study, we report the first detailed description of the harassment strategy used by European Starlings to force a breeding pair of Green-barred Woodpecker to abandon its cavities in an urban area of central-eastern Argentina. In addition, because we were able to monitor the breeding pair throughout the entire breeding season, we provide information on the breeding cost for the Green-barred Woodpecker caused by the European Starling.
Methods
Study area
The study was carried out in the city of La Plata, in northern Buenos Aires province, Argentina (34° 55’ S, 57° 57’ W, 0 m a.s.l.). The study site is located in the Pampas ecoregion [Citation35] and has a humid temperate climate, with an average annual temperature of 15 °C and an average annual rainfall greater than 800 mm [Citation36]. Based on adult activity during the monitored breeding season, the Green-barred Woodpecker breeding territory was a patch of forest of ~1 ha located in a park within a highly trafficked area of the city. The breeding territory was mainly dominated by trees of Populus sp. and Eucalyptus sp.
Study species
The Green-barred Woodpecker is a medium-sized (24–28 cm, 104–160 g) omnivorous bird with an ant-based diet [Citation37,Citation38]. It is present in humid subtropical forests, transition forests, savannas, and open areas from northeast Brazil to center-south Argentina [Citation37,Citation39]. It nests in cavities excavated by both members of the breeding pair in branches or trunks of trees (AJ, pers. obs.). In Argentina its breeding season lasts from mid-September to early-January [Citation26,Citation37].
The European Starling is a medium-sized (21–22 cm; 58–100 gr) [Citation15] omnivorous passerine, which feeds on arthropods, fruits, and grains [Citation40]. It is native to Europe, southwest Asia, and north Africa [Citation15], and is currently distributed across every continent except Antarctica [Citation15,Citation40]. It has a noticeable ability to adapt and reproduce in urban and peri-urban areas, forming flocks of thousands of individuals [Citation41]. Its breeding season in the study area lasts from early-September to late-December [Citation15,Citation26].
Field procedure
We observed seven breeding attempts within the confines of a breeding territory of a Green-barred Woodpecker pair between September 2021 and January 2022. Although the breeding pair was not banded, we assumed nest attempts belonged to the same breeding pair because: 1) a relatively small size of the breeding territory monitored and the territorial behavior of the Green-barred Woodpecker [Citation42], 2) we never observed more than two adults in the area, and 3) we never observed two active nests simultaneously (). We found the nests while walking the breeding territory, through observation of the breeding adults’ behavior. We found all of the nests during the construction stage, directly observing the adults expelling wood chips from the cavity [see [Citation7]]. Each breeding attempt was monitored every 2–3 days. To avoid disturbances, we did not climb trees to access the nest content, and we estimated the nesting stage by observing the cavity entrance for 30–45 min with binoculars at each visit. During this time, we recorded the activity of adults and/or starlings. When we detected interactions with starlings, we expanded the observation time to 90 minutes (in order to increase the chances of detecting an interaction), using binoculars and a video camera (Handycam Sony HDR-CX330) placed at a distance of ~10 m directly facing the cavity entrance. In most nests we estimated the start date of nest construction based on the degree of completion of the entrance hole when it was found (average nest construction time for our breeding pair: ~5 days, see Results). In the few cases (N = 2 nests) where it was not possible, we estimated it by considering the abandonment date of the last nest and the stage in which the new nest in the same breeding territory was found. We considered a nest in the “egg stage” (egg laying or incubation) when at least 5 days had elapsed since the start of construction and one of the adults remained inside the cavity (without observing wood chip extraction) during the observation period. We considered a nest in the “nestling stage” when at least 18 days had elapsed from the start date of construction (5 days of construction, 2 days of laying for a bird with asynchronous hatching, and 11 days of incubation; AJ, unpubl. data), or when one of the adults remained in the nest (possibly brooding) and the other adult made short and frequent visits to the nest delivering food to the nestlings. We considered a nest abandoned when no adult woodpecker activity was observed in the nest surroundings for at least two consecutive visits, or when we observed starlings using the cavity. Finally, a nest was considered successful if we observed the adults feeding/assisting a fledgling within the breeding territory.
Table 1. Nest monitoring of a breeding pair of Green-barred Woodpecker (Colaptes melanochloros) in an urban area of La Plata, Argentina, during the 2021–2022 breeding season. Dates of the beginning of nest construction and egg laying are indicated and the type of interaction with the European Starling (Sturnus vulgaris) is detailed.
Results
We recorded seven consecutive breeding attempts between September and December 2021, all of them in new cavities excavated by the woodpecker breeding pair (nest tree-support features are detailed in ). On average, nests took 5.2 ± 0.6 days to excavate (range = 4–7 days, N = 5 nests). In all breeding attempts except for the last, we observed interactions with starlings during the days after cavity excavation began (). None of the breeding attempts were successful: three were abandoned late in the construction stage, two during the egg laying stage, and two during the nestling stage (in the latter two cases, with no apparent cause for abandonment, ). In the first five breeding attempts (September–October) starlings used the cavity to build their own nest after the woodpeckers abandoned it.
Table 2. Features of the nest tree-support of a breeding pair of Green-barred Woodpecker (Colaptes melanochloros) in a urban area of La Plata, Argentina, during the 2021–2022 breeding season.
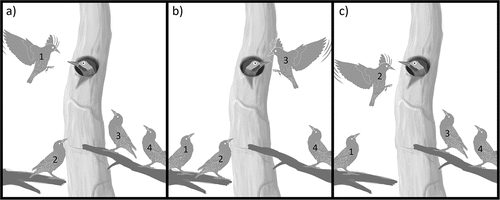
We recorded two types of interaction with starlings: a) 1–2 starlings perched near the cavity (i.e. less than 50 cm from the nest entrance) without evidence of agonistic behaviors () and b) 4–6 starlings perched near the cavity showing cooperative harassment behaviors ( and Suppl. Material S1). Cooperative harassment occurred at three nests (), once in each nest, and consisted of individual intimidatory approach flights in front of the cavity entrance ( and Suppl. Material S1). This flight (“hovering”) was sustained for ~5 seconds in front of the entrance hole and was followed by repeated hovering by another individual ( and Suppl. Material S1). During this event, the woodpecker remained inside the cavity entrance facing the approaching starlings, as detailed in . This cooperative harassment continued for 15, 17 and 26 min each, resulting in either the Green-barred Woodpecker flushing and subsequent entry of the starling into the cavity (N = 2 nests) or the desertion of the group of starlings (N = 1 nest). Although in the latter case the Green-barred Woodpecker remained inside the nest during the observation period, the nest was abandoned the next day.
Discussion
We provide evidence of interactions between the Green-barred Woodpecker, a native primary cavity nesting bird of temperate forests of the southern cone of South America, and the European Starling, an invasive bird that uses cavities for breeding. This information is especially relevant at a time of accelerated expansion of the invasive species within the Argentine territory [Citation43,Citation44]. Although the adverse effects to native fauna caused by the European Starling is known worldwide [Citation24,Citation27,Citation28,Citation30,Citation45,Citation46], in the Neotropics there are still few reports of interactions with native birds [Citation26,Citation29,Citation31,Citation32]. Studies in other regions of the world have reported that harassment by European Starlings towards breeding birds increases the likelihood of nest abandonment and usurpation [Citation28,Citation30], although there are no details on the cooperative strategy used. We report for the first time a detailed description of the harassment strategy used by European Starlings to force woodpeckers to abandon the cavity. Interestingly, our breeding pair never showed an active defense of the cavity against the European Starlings [but see [Citation26]], but remained passively inside the cavity. In addition, we highlight the harassment strategy effectiveness, since the starlings forced woodpeckers nest abandonment (and built their own nests) in five consecutive woodpecker breeding attempts.
Because we were able to monitor seven reproductive attempts of the Green-barred Woodpecker pair throughout an entire breeding season, we found evidence of the high reproductive cost of interactions with European Starlings. Worryingly, none of the seven nesting attempts were successful and the breeding season ended without recruitment of new individuals to the local population pool. We are confident that the first five attempts failed due to the interactions with the European Starlings, forcing the breeding pair of Green-barred Woodpeckers to excavate new cavities until the end of the breeding season. This scenario caused at least two well-defined damages for the breeding pair of Green-barred Woodpeckers. First, the energy cost of excavating new cavities [Citation34] and producing eggs for each breeding attempt [Citation47,Citation48] has detrimental effects on the female body condition [Citation49]. In this particular breeding pair of Green-barred Woodpeckers, we believe it was possible to excavate such a large number of new cavities (in such a short time) because the available wood was soft [mean dry wood density of Populus sp. 0.42 g/cm3, see Citation50], but the investment could be considerably higher in forests without this condition. Second, the constant interruptions of breeding attempts delayed the initiation date of reproduction for the Green-barred Woodpecker pair, which had at least two consequences: 1) as the breeding season progresses, the probability that woodpeckers will make new nesting attempts decreases [Citation51] and 2) at these latitudes, the nest success rate decreases as the season progresses [Citation52–54, AJ, unpubl. data], because of a higher rate of nest predation or seasonal variation in food availability [Citation30]. For these reasons, by causing the abandonment of nests and delaying their reproduction, the European Starling indirectly reduces the reproductive success of the Green-barred Woodpecker.
Competition with the European Starling during the breeding season has been observed to exert selection for delayed breeding in several woodpecker species [Citation14,Citation26]. However, because of the low nest success rate of the Green-barred Woodpecker late in the season, a phenology shift to avoid competition may not be possible. Currently, the Green-barred Woodpecker population in this region of the Neotropics is not threatened [Citation39], but if we extrapolate this cooperative agonistic behavior of the European Starling to the reproductive habitats of cavity nesting species with a threat category, the consequences could be very serious. In addition, this type of agonistic behavior would not only affect cavity excavators, but also a wide variety of secondary cavity nesters (especially medium-small sized ones) that breed and compete with the European Starling for cavity use [Citation55]. Considering the rapid expansion of the European Starling in Argentina [Citation43,Citation44] and the competitive threat it represents for native cavity-nesting birds [Citation17,Citation24,Citation26,Citation31,Citation44,Citation56, this study], in addition to the limited supply of cavities in the breeding habitats of many birds [Citation3,Citation4], we alert the governmental authorities and environmental NGOs to take urgent measures to control the populations of this aggressive invasive species.
Supplemental Material
Download MP4 Video (27.7 MB)Acknowledgments
We are grateful to R. Ramos for help with data collection, nest monitoring and helpful comments to a previous version of this manuscript. We appreciate the improvements in English writing made by K. Depot. This paper is the Scientific Contribution N° 1231 of the Institute of Limnology “Dr. Raúl A. Ringuelet” (ILPLA, CCT-La Plata CONICET, UNLP).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/23766808.2022.2145089
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Monterrubio-Rico TC, Escalante-Pliego P. Richness, distribution and conservation status of cavity nesting birds in Mexico. Biol Conserv. 2006;128(1):67–78.
- Cockle KL, Martin K, Wesołowski T. Woodpeckers, decay, and the future of cavity-nesting vertebrate communities worldwide. Front Ecol Environ. 2011;9(7):377–382.
- Newton I. The role of nest sites limiting the number of hole-nesting birds: a review. Biol Conserv. 1994;70(3):265–276.
- Cockle KL, Bodrati A, Lammertink M, et al. Cavity characteristics, but not habitat, influence nest survival of cavity-nesting birds along a gradient of human impact in the subtropical Atlantic forest. Biol Conserv. 2015;184:193–200.
- Stojanovic D, Nee Voogdt JW, Webb M, et al. Loss of habitat for a secondary cavity nesting bird after wildfire. For Ecol and Management. 2016;360:235–241.
- Politi N, Hunter M, Rivera L. Assessing the effects of selective logging on birds in neotropical piedmont and cloud montane forests. Biodivers and Conserv. 2012;21(12):3131–3155.
- Jauregui A, Rodríguez SA, González García LN, et al. Wood density and tree size used as cues to locate and excavate cavities in two Colaptes woodpeckers inhabiting a threatened southern temperate forest of Argentina. For Ecol and Managm. 2021;502:119723.
- Newton I. Population limitation in birds. London(LND): Academic Press; 1998.
- Gibbons P, Lindenmayer DB. Tree hollows and wildlife conservation in Australia. Melbourne: CSIRO Publishing; 2002.
- Cockle KL, Martin K, Drever MC. Supply of tree-holes limits nest density of cavity nesting birds in primary and logged subtropical Atlantic forest. Biol Conserv. 2010;143(11):2851–2857.
- Cockle KL, Martin K, Wiebe K. Selection of nest trees by cavity-nesting birds in the neotropical Atlantic forest. Biotropica. 2011;43(2):228–236.
- Lorenz T, Vierling KT, Johnson TR, et al. The role of wood hardness in limiting nest site selection in avian cavity excavators. Ecol Applic. 2015;25(4):1016–1033.
- Pell AS, Tidemann CR. The impact of two exotic hollow-nesting birds on two native parrots in Savannah and woodland in eastern Australia. Biol Conserv. 1997;79(2–3):145–153.
- Koenig WD. European starlings and their effect on native cavity‐nesting birds. Conserv Biol. 2003;17(4):1134–1140.
- Craig AJ, Feare CJ. Family Sturnidae (starlings). In: Del Hoyo J, Elliot A, Christie DA, editors. Handbook of the birds of the world, Vol.14, Bush-shrikes to old world sparrows. Barcelona (BCN): Lynx Editions; 2009. p. 654–758.
- Navas J. Las aves exóticas introducidas y naturalizadas en la Argentina. Rev del Museo Argentino de Cienc Nat nueva serie. 2002;4(2):191–202.
- Di Giacomo AG, Di Giacomo AS, Barbarskas M. Nuevos registros de Sturnus vulgaris y Aeridotheres cristatellus. Nuestras aves. 1993;29:32–33.
- Isacch JP, Isacch J. Estornino Pinto (Sturnus vulgaris) en la ciudad de Mar del Plata (provincia de Buenos Aires, Argentina). Nuestras Aves. 2004;47:33.
- Jensen FR. Nuevos registros de Estornino Pinto (Sturnus vulgaris) para el sureste de la provincia de Entre Ríos, Argentina. Nuestras Aves. 2008;53:22.
- Klavins J, Álvarez D. El Estornino Pinto (Sturnus vulgaris) en la provincia de Córdoba, Argentina. Nuestras Aves. 2012;57:27–29.
- Zanotti M. Presencia del Estornino Pinto (Sturnus vulgaris) en la provincia de Mendoza, Argentina. Nuestras Aves. 2013;58:5–7.
- Lowe S, Browne M, Boudjelas S, et al. 100 of the world’s worst invasive alien species: a selection from the global invasive species database. Auckland (NZ): Auckland: Invasive Species Specialist Group; 2000.
- Santiago-Alarcon D. Delgado-V CA. warning! Urban threats for birds in Latin America. In: MacGregor-Fors I, Escobar-Ibáñez JF, editors. Avian ecology in Latin American cityscapes. Cham: Springer; 2017. p. 125–142.
- Weitzel NH. Nest-site competition between the European starling and native breeding birds in northwestern Nevada. Condor. 1988;90(2):515–517.
- González-Oreja JA, Zuria I, Carbó-Ramírez P, et al. Using variation partitioning techniques to quantify the effects of invasive alien species on native urban bird assemblages. Biol Invasions. 2018;20(10):2861–2874.
- Jauregui A, Gonzalez E, Segura LN. Impacts of the invasive European starling on two neotropical woodpecker species: agonistic responses and reproductive interactions. Emu-Austral Ornithol. 2021;121(3):223–230.
- Kerpez TA, Smith NS. Competition between European starlings and native woodpeckers for nest cavities in saguaros. Auk. 1990;107(2):367–375.
- Frei B, Nocer JJ, Fyles JW. Interspecific competition and nest survival of the threatened red-headed woodpecker. J of Ornithol. 2015;156(3):743–753.
- Zárate V, Juncosa Polzella AS. Usurpación de nido de carpintero del cardón (Melanerpes cactorum) por parte de estornino pinto (Sturnus vulgaris). Nuestras Aves. 2020;65(1):58–60.
- Ingold DJ. Delayed nesting decreases reproductive success in northern flickers: implications for competition with European starlings. J of F Ornithol. 1996;67(2):321–326.
- Rebolo-Ifrán N, Fiorini VD. European starling (Sturnus vulgaris): population density and interactions with native species in Buenos Aires urban parks. Ornitol Neotrop. 2010;21(4):507–518.
- Ibáñez LM, Girini JM, Palacio FX, et al. Interacciones entre el estornino pinto (Sturnus vulgaris) y aves nativas de Argentina por el uso de cavidades. Revista Mexicana de Biodivers. 2017;88(2):477–479.
- Diamond JM, Ross MS. Overlap in reproductive phenology increases the likelihood of cavity nest usurpation by invasive species in a tropical city. Condor. 2020;122(3):1–13.
- Wiebe KL, Koenig W, Martin K. Costs and benefits of nest reuse versus excavation in cavity-nesting birds. Ann Zool Fennici. 2007;44:209–217.
- Brown A, Pacheco S . Propuesta de actualización del mapa ecorregional de la Argentina. In: Brown A, Martinez-Ortiz U, Acerbi Meditors. Situación Ambiental Argentina 2005. Buenos Aires (BA): Fundación Vida Silvestre Argentina; 2006. p. 25–32.
- Estadísticas de precipitaciones [Internet]. Argentina: instituto Nacional de Tecnología Agropecuaria, Sistema de Información sobre Clima y Agua; 1960-2011 [cited 2022 Jan 22]. Available from 2022 Jan 22: http://climayagua.inta.gob.ar/estad%C3%ADsticas_de_precipitaciones
- Winkler H, Christie DA. Family Picidae (woodpeckers). In: Del Hoyo J, Elliot A, Sargatal J, editors. Handbook of the birds of the world. Lynx Editions. Vol. 7, Barcelona (BCN): Jacamars to Woodpeckers; 2002. p. 296–555.
- Beltzer AH, De Amsler GP, Neffen MI. Biología alimentaria del carpintero real Colaptes melanochloros (Aves: Picidae) en el valle aluvial del río Paraná, Argentina. An de Biología Servicio de Publicaciones de la Universidad de Murcia. 1995;20:53–59.
- BirdLife international species factsheet: Colaptes melanochloros [Internet]. IUCN Red List for birds. 2022 [cited 2022 Jan 27]. Available from 2022 Jan 27: http://datazone.birdlife.org/species/factsheet/22727950
- Cabe PR. In: Billerman SM European Starling (Sturnus vulgaris), editor. Birds of the world; 2022. https://birdsoftheworld.org/bow/species/eursta/1.0/introduction
- Petracci PF, Delhey KJ, Pérez CH, et al. Nuevos aportes al conocimiento de la distribución y anidación de algunas especies de aves en la Argentina. Nuestras Aves. 2004;48:25–31.
- Dias RI, Webster MS, Goedert D, et al. Cooperative breeding in the campo flicker I: breeding ecology and social behavior. Condor. 2013;115(4):847–854.
- Zufiaurre E, Abba A, Bilenca D, et al. Role of landscape elements on recent distributional expansion of European starlings (Sturnus vulgaris) in agroecosystems of the Pampas, Argentina. Wilson J of Ornithol. 2016;128(2):306–313.
- Liébana MS, Santillán MA, Seen NP, et al. Aportes al conocimiento de la distribución y biología del Estornino Pinto (Sturnus vulgaris) en el centro de Argentina. Acta Zoológica Lilloana. 2020;64(1):43–57.
- Smith KW. Has the reduction in nest-site competition from Starlings Sturnus vulgaris been a factor in the recent increase of great spotted woodpecker dendrocopos major numbers in Britain? Bird Study. 2005;52(3):307–313.
- Blackburn TM, Lockwood JL, Cassey P. Avian invasions: the ecology and evolution of exotic birds. Oxford (NY): Oxford University Press; 2009.
- Monaghan P, Nager RG. Why don’t birds lay more eggs? Trends in Ecol & Evol. 1997;12(7):270–274.
- Christians JK. Avian egg size variation within species and inflexibility within individuals. Biol Rev. 2002;77(1):1–26.
- Monaghan P, Nager RG, Houston DC. The price of eggs: increased investment in egg production reduces the offspring rearing capacity of parents. Biol Sciences. 1998;265(1407):1731–1735.
- Atencia MD. Densidad de Maderas (kg/m3) [Internet]. Argentinian Government Report. 2003 cited 2022 Jan 29]. Available from 2022 Jan 29: https://www.inti.gob.ar/publicaciones/descargac/365.
- Wiebe KL. Delayed timing as a strategy to avoid nest-site competition: testing a model using data from starlings and flickers. Oikos. 2003;100(2):291–298.
- Segura LN, Berkunsky I. Nest survival of the Red-crested Cardinal (Paroaria coronata) in a modified habitat in Argentina. Ornitol Neotrop. 2012;23(4):489–498.
- Segura LN, Mahler B, Berkunsky I, et al. Nesting biology of the red-crested cardinal (Paroaria coronata) in south temperate forests of central Argentina. The Wilson J of Ornithol. 2015;127(2):249–258.
- Gonzalez E, Jauregui A, Segura LN. Breeding biology of the yellow-browed tyrant (Satrapa icterophrys) in south temperate forests of central Argentina. The Wilson J of Ornithol. 2019;131(3):534–542.
- der Hoek Y V, Gaona GV, Ciach M, et al. Global relationships between tree-cavity excavators and forest bird richness. R Soc Open Sci. 2020;7(7):192177.
- Rizzo F. Utilización de nidos de Hornero (Furnarius rufus) por el Estornino pinto (Sturnus vulgaris). Nuestras Aves. 2010;55:33–35.