ABSTRACT
The white-lipped peccary (Tayassu pecari) is considered a key species for its role as an ecosystem engineer. Given their important ecological function, there is a great concern in the scientific community regarding the many reports of disappearances or great abundance reductions throughout its distribution (from southern Mexico to northern Argentina). Based on an extensive survey effort, we report new data of presence of WLP after a period of no detections in the Argentine Yungas. The study was conducted in the Yungas ecoregion, provinces of Jujuy and Salta, Argentina. Data was collected from camera trap, direct sightings and footprints during 2013–2021. From a total of 30,186 trap nights, we obtained 8 WLP detections. There were no detections before 2017, while as of 2018 there was at least one camera trap record in each of the years. Additionally, opportunistic records were obtained yearly from 2019 to 2021. The period without detections could be related to a period of a population cycle, as suggested in different regions of America during the last century, being disease the most likely cause. These cycles generally follow a pattern of a rapid population decline, a period of absence or low abundance followed by slow population growth. We believe that if the trend continues and the number of detections increases, we could be facing the early stages of the increase phase in the WLP population cycle in the Argentine Yungas. This type of report is important to document and contribute to a better understanding of the WLP population cycle process.
Introduction
Some species have a key role in the ecosystem, affecting its function and structure. The disappearance of those species is a very concerning conservation threat since had a great impact causing loss of ecosystems’ structure, functions and resilience [Citation1,Citation2]. For example, there have been reports showing alterations in seed dispersal and predation, plant functional traits, primary productivity, nutrient cycling and carbon storage caused by the loss of herbivorous mammals in the Neotropics [Citation3–6].
Because of their role as ecosystem engineers, white-lipped peccary (Tayassu pecari) are considered important species for ecosystem functioning [Citation7,Citation8]. Some of the effects of white-lipped peccary (WLP) in their environment include different actions such as dispersing seeds and mycorrhizae spores, predation (plants and animals), and creation of disturbances that plants and animals colonize, besides being important prey items for large felines [Citation7–10]. Due to the species’ high biomass, these consequences are magnified [Citation7], because they can weigh up to 50 kg and form social groups of 50 to 100 individuals, although there are anecdotal reports of herds of more than 1000 individuals [Citation11–13].
The species distribution extends from the south of Mexico to the north of Argentina. Because they need wide areas to survive, they are extremely vulnerable to habitat loss, fragmentation and poaching [Citation9]. They are internationally categorized as Vulnerable [Citation14] and Endangered in Argentina [Citation15], and in both cases, a population reduction tendency was the main criteria for the categorization. There have been reports of disappearances or great abundance reductions throughout all its distribution [Citation12,Citation16,Citation17], including our study area, Argentinean Yungas [Citation18], where records used to be common [Citation19,Citation20].
In an effort to document the WLP possible reappearance in the northern part of Argentinean Yungas, we are reporting new data on the occurrence of the species, after an extended period of no detections.
Methods
Study site- Surveys were conducted in the Yungas ecoregion, north of 24S parallel, provinces of Jujuy and Salta, Argentina (). The Yungas subtropical forest occurs in the eastern slopes of the Andes, from 400 to 2300 meters of altitude, representing the most extensive subtropical forest ecosystem in the country [Citation21]. The area presents a high diversity and an important degree of endemism e.g., [Citation22–24] and provides important ecological services for a vast region [Citation25].
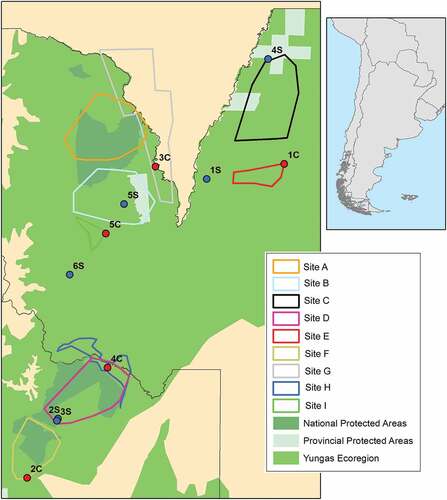
Figure 1. Map of the study area with colored polygons denoting the regions sampled during camera trap surveys. Different shades of green are used to represent the Yungas ecoregion, National s and Provincial Protected Areas.
Climate is subtropical with a dry season (April to October), an average annual temperature of 21°C and annual rainfall varies from 700 to 2000 mm and increasing with altitude [Citation25]. Three altitudinal strata can be differentiated [Citation26]: Premontane Rainforest (400 to 900 m), Montane Rainforest (900–1600 m), and Clouded Montane Forest (1600–2300 m). Each of them shows specific characteristics in weather, flora and fauna.
The area sampled is a mosaic of protected areas and silvopastoral lands with different levels of usage intensity. There are some agricultural lands, mostly in the lower regions, and are some small villages, towns and cities, but the area is sparsely populated as the larger settlements are near the borders.
Camera trap surveys- From 2013 to 2021 we carried out camera trap surveys in 8 sites (). Cameras were installed in animal trails and waterways at 30 cm from the ground and set to take pictures all day. Most surveys sites consisted in a mosaic of protected and non-protected areas and with an altitude ranging from 434 to 1723 m above sea level.
Opportunistic sightings- We performed many single and multiple-day hikes into the forests to install camera traps and other activities. During those walks, we directly observed WLP or their footprints. Although we do not account for sampling effort, we believe that it was similar in the period 2013–2019 and somehow reduced from 2020 because of the COVID-19 pandemics restrictions.
Data analysis- Sampling effort (trap nights) was calculated as the sum of days that each camera was operational for a complete 24-h period. We calculated the capture rate for each camera trap site as the number of detections/1000 trap nights and the percentage of camera traps on each site that detected the species. For each WLP record, we used remote sensing to calculate distances to the nearest protected area, town, road (including paved and secondary dirt roads), paved road and altitude.
Results
The sampling effort of the 219 camera traps was allocated on the different sites as shown in . From a total of 30,186 trap nights, we obtained eight 8 WLP detections (). No WLP was detected before 2017 (Site E), but the species was detected at least once in each of the subsequent years. In most of the independent sites, WLP were detected only once except for the Site E, where WLP were detected three times in the same camera trap (11 and 1 days apart) and Site F, where they were detected twice in the same camera trap (122 days apart). For each site, detection rate (detections/1000 trap nights) ranged from 0.09 to 0.53 and the percentage of the camera trap sites with WLP detection ranged from 2.22 to 16.67.
Figure 2. Study area map showing WLP reported camera trap records (red dots), sightings/footprints (blue dots). Labels on dots correspond to record identification. Map shows international borders (light blue lines), main paved roads (yellow lines) and main cities (purple squares).
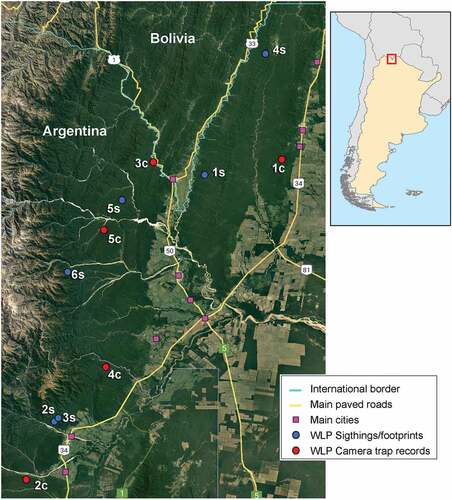
Table 1. Camera trap surveys and white-lipped peccary (WLP) detected in Argentinean Yungas. Survey year and ID, number of sampling points (camera traps), sampling effort expressed as the sum of the number of nights that each camera trap was active, detection rate as WLP detections/1000 trap days and percentage of camera traps where WLP was detected in the site.
No direct observation records or footprints were observed during the period 2013–2016 in the study area. From 2019 to 2021 in three points we observed footprints and in four points we directly observe WLP (, ). In 2019, we recorded several groups of footprints dispersed over at least 3 linear kilometers on a stream bank, and we observed a group of at least six WLP individuals (Sighting 1S, , ). During the same year, a set of footprints were found in Calilegua National Park, then several footprints groups were found repeatedly during 2019 and 2020 in a nearby location (Sightings 2S and 3S), outside the protected area. Two additional WLP groups were sighted near Acambuco and Pintascayo provincial protected areas, in Salta province, in 2020 and 2021, respectively (Sightings 4S and 5S, , ). Finally, a small herd was seen by local people, and one of the individuals was killed by dogs (Sightings 6S, , ). The location of the WLP detections is detailed in the Supplemental Material. Considering all detection methods, only two WLP detections were inside protected areas, one in Calilegua National Park and the other in El Pantanoso Private Reserve. The mean distance (±SD, range) to protected areas was 12.52 km (±13.59, 0–38.2). The mean distances to paved or closest road and the nearest town were 16.43 km (±12.29, 1.46–45.02), 3.02 km (±3.41, 0.38–10.31) and 13.04 km (±6.52, 5.02–26.39), respectively, while the mean altitude was 733.09 m above sea level (±194.61, 423–1107).
Table 2. White-lipped peccary opportunistic records in Argentinean Yungas. Record identification (Record ID), year, type of sign and observations.
Discussion
In this study, we reported a new set of white-lipped peccary records from different sources including camera trapping surveys and opportunistic observations (both direct and footprints) from the 2017 to 2021 period, after a great period of absence of detections.
The study area was designated as WLP conservation unit 37 [Citation19] and was thought to have a good long-term survival probability. Although not considered rare in the past, e.g. [Citation19,Citation20,Citation27,Citation28] and despite the important sampling effort, we did not detect any WLP in the northern part of Argentinean Yungas from 2013 to 2017. After that, we slowly started to record WLP in different locations yearly until the present. Similarly, only one record was obtained by Bardavid et al. [Citation18], during the period 2016–2017 surveying with camera traps in approximately the same region.
Some species are subjected to periodic fluctuations in abundances, known as population cycles. Those cycles, generally follow a pattern of a rapid population decline, a period of absence or low abundance followed by a slow population growth until the peak is reached and the cycle starts over. Animal population cycles have been studied extensively but, in many cases, underlying processes are still poorly understood [Citation29,Citation30]. Some of the species that have been reported to show population cycles are snowshoe hares, voles and lemmings, forest Lepidoptera, red grouse and WLP; with cycle lengths varying from three years to decades [Citation12,Citation30]. Natural drivers of population cycles include predators, parasitoids, pathogens, detrimental conditions, or a combination of them, leading to a reduction of reproduction and/or increase mortality. Also, anthropogenic causes have been proposed such as climate change, habitat fragmentation and direct killing [Citation12,Citation30].
Population cycles in WLP had been recorded in different regions of America for almost a century (see [Citation12] for a detailed description) and are part of indigenous people’s knowledge in some regions [Citation31]. The reported disappearance or low abundance period can vary between 7 and 12 years, 20 in cases of high habitat fragmentation and human pressure. The proposed causes to explain the declines in WLP populations across different regions are migration, overhunting and disease. Although a major decline in populations has recently been observed in Mesoamerica [Citation17,Citation32], caused by a combination of habitat loss and overhunting, disease is the most supported cause for South America [Citation12,Citation16,Citation33]. This is because there are several ecological and behavioral characteristics that make WLP susceptible to rapid disease spread, such as living in big herds, low territoriality and herd overlapping [Citation7,Citation11,Citation16]. Therefore, it would be reasonable to believe that the pattern found may be a manifestation of the population cycle caused by disease, as hypothesized previously for the rest of South America [Citation12]. At least, we have no reason to suspect that the decline was caused by increased hunting (no increase in poaching records, either direct sighting, news, or camera traps), habitat loss, or migration.
Although more data is needed, we believe that if the tendency continues, we might be in front of the early stages in the increase phase in the WLP population cycle. The extensiveness of the area in addition to the numerous areas of high difficulty of access makes it likely that an undetected small number of WLP herds persisted and originated the population increment and consequent increment in detections. Our reported data are widely distributed in the study area, covering a wide range of altitudes (and all three Yungas vegetation floors) and exposure to human disturbances (roads and towns), suggesting no recolonization pattern. The species was recorded on different types of land use, from strictly protected areas to places located near towns and likely to be exposed to poaching. These records on non-protected areas and close to human settlements and roads (including two with multiple detections) reinforce the hypothesis that cycles may not be caused by human activities.
It would be unsound to attribute a relationship between detection rate and abundance given the limited number of detections and the possibility of a differential detection probability between sampling sites. Considering that, is interesting to note that the detection sites recorded on surveys with the greatest detection rates (Survey E and F), are both located in non-protected areas and at low distances from cities and main roads. This might be indicating the mentioned low consistency in detection rate given the low number of detections (e.g. a difference in one detection can lead to a great change in detection rate), or the presence of another variable that we do not evaluate that is playing a more important role. Similarly, we did not detect any temporal tendency in the detection rate, which can indicate an increase in population numbers during sampled period. This can have the same explanation as the comparison between sites and/or might be indicating that the recovery is very slow to be recorded in such a short period of time.
Is critical to keep gathering data and protect the emerging populations, since at this stage, probably still in low population numbers, poaching might have a great influence in slowing down or preventing the population recovery of WLP in Argentinean Yungas. This type of report is important to document and contribute to a better understanding of the WLP population cycle process. Furthermore, these pieces of information would be an aid to species conservation status evaluation and hopefully, will help policymakers to design informed actions to protect the species.
Geolocation information
The study was conducted in the Yungas ecoregion, north of the 24S parallel, provinces of Jujuy and Salta, Argentina. The approximate center of the area is 23° 07ʹS, 64° 42ʹW.
Acknowledgments
We thank three anonymous reviewers for their comments that substantially improved the quality of this manuscript. We are grateful to Ricardo Ayarde, Hugo Ortíz, Ariel Ayarde, Diómedes Garay, Bidoldo Ortiz, Gloria Ramos y Adalberto Izaurralde, Marcelo Gallegos, Luis Correa, Martín Correa and all the park rangers, volunteers and local people who contributed to data collection. We acknowledge Administración de Parques Nacionales - Delegación Regional Noroeste (APN-DROA), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Fundación Biodiversidad Argentina, Fundación CEBIO, Ministerio de Ambiente de Jujuy and Secretaría de Ambiente y Desarrollo Sustentable de Salta, Servicio Nacional de Áreas Protegidas de Bolivia (SERNAP) and Protección del Medio Ambiente de Tarija (PROMETA) for endorsing and authorizing the project. Our project received support from WildCRU, Fondation Segré, Rufford Foundation, Word Land Trust, PPD Argentina-PNUD, Born Free, APN-DROA. JIR and FC received financial support from CONICET and PGP from APN-DROA.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Schmid B, Balvanera P, Cardinale BJ, et al. Consequences of species loss for ecosystem functioning: meta-analyses of data from biodiversity experiments. In: Naeem S, Bunker DE, Hector A, et al. editors. Biodiversity, ecosystem functioning, and human wellbeing. Oxford University Press, Oxford. 2009; p. 14–29.
- Oliver TH, Isaac NJB, August TA, et al. Declining resilience of ecosystem functions under biodiversity loss. Nat Commun. 2015;6(1):10122.
- Wright SJ, Zeballos H, Domínguez I, et al. Poachers alter mammal abundance, seed dispersal, and seed predation in a Neotropical forest. Conserv Biol. 2000;14(1):227–239.
- Kurten EL, Wright SJ, Carson WP. Hunting alters seedling functional trait composition in a Neotropical forest. Ecology. 2015;96(7):1923–1932.
- Peres CA, Emilio T, Schietti J, et al. Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proc Natl Acad Sci USA. 2016;113(4):892–897.
- Forbes ES, Cushman JH, Burkepile DE, et al. Synthesizing the effects of large, wild herbivore exclusion on ecosystem function. Funct Ecol. 2019; 33(9):1597–1610.
- Altrichter M, Sáenz JC, Carrillo E, et al. Dieta estacional del Tayassu pecari (Artiodactyla: tayassuidae) en el Parque Nacional Corcovado, Costa Rica. Rev Biol Trop. 2000;48(2–3):689–702.
- Beck H, Thebpanya P, Filiaggi M. Do neotropical peccary species (Tayassuidae) function as ecosystem engineers for anurans? J. Trop. Ecol. 2010;26(4):407–414
- Altrichter M, Taber A, Beck H, et al. Range-wide declines of a key neotropical ecosystem architect, the near threatened white-lipped peccary tayassu pecari. Oryx. 2012;46(1):87–98.
- Cavalcanti SMC, Gese EM. Kill rates and predation patterns of jaguars (Panthera onca) in the southern Pantanal, Brazil. J Mammal. 2010;91(3):722–736.
- Sowls LK. Javelinas and other peccaries: their biology, management and use. Texas A&M University Press; 1997.
- Fragoso JMV, Antunes AP, Silvius KM, et al. Large-scale population disappearances and cycling in the white-lipped peccary, a tropical forest mammal. PLoS ONE. 2022;17(10):e0276297.
- Taber AB, Novaro AJ, Neris N, et al. The food habits of sympatric jaguar and puma in the Paraguayan Chaco. Biotropica. 1997;29(2):204.
- Keuroghlian A, Desbiez ALJ, Reyna-Hurtado RA, et al. Tayassu pecari. The IUCN red list of threatened species 2013. 2013;eT41778A44051115. https://www.iucnredlist.org/species/41778/44051115
- de Bustos S, Varela D, Lizarraga L, et al. Tayassu pecari, SAyDS–SAREM, editor. Categorización 2019 de los mamíferos de Argentina según su riesgo de extinción Lista Roja de los mamíferos de Argentina Versión digital. 2019; https://cma.sarem.org.ar/es/especie-nativa/tayassu-pecari
- A long-term study of white-lipped peccary (Tayassu pecari) population fluctuations in northern amazonia: anthropogenic vs. “natural” causes. In Silvius K, Bodmer R, Fragoso J, editors. People in nature, 18nd. Columbia University Press, New York. 2004; p. 286–296.
- Thornton D, Reyna R, Perera-Romero L, et al. Precipitous decline of white-lipped peccary populations in Mesoamerica. Biol Conserv. 2020;242:108410.
- Bardavid S, de Bustos S, Politi N, et al. Escasez de registros de pecarí labiado (Tayassu pecari) en un sector de alto valor de conservación de las Yungas Australes de Argentina. Mastozool Neotrop. 2019;26(1):167–173.
- Taber A, Chalukian SC, Altrichter M, et al. El destino de los arquitectos de los bosques neotropicales: evaluación de la distribución y el estado de conservación de los pecaríes labiados y los tapires de tierras bajas. 2008. p. 182.
- Perovic PG Ecología de la comunidad de félidos de las selvas nubladas del noroeste argentino. [ dissertation]. Córdoba; Universidad Nacional de Córdoba, 2002.
- Entrocassi GS, Gavilán RG, Sánchez-Mata D. Subtropical mountain forests of las yungas: vegetation and bioclimate. Switzerland: Springer Nature; 2020.
- Brown AD, Blendinger PG, Lomáscolo T, et al. editors. Selva pedemontana de las Yungas: historia natural, ecología y manejo de un ecosistema en peligro. Tucumán Argentina: Ediciones del Subtrópico. 2009.
- Navarro FR, Cuezzo F, Goloboff PA, et al. Can insect data be used to infer areas of endemism?: An example from the Yungas of Argentina. Rev Chil Hist Nat. 2009;82(4):507–522.
- Sandoval ML, Szumik CA, Barquez RM. Bats and marsupials as indicators of endemism in the Yungas forest of Argentina. Zool. Res. 2010;31:12.
- Malizia L, Pacheco S, Blundo C, et al. Caracterización altitudinal, uso y conservación de las Yungas Subtropicales de Argentina. Ecosistemas. 2012;21:53–73.
- Cabrera AL. Regiones Fitogeográficas Argentinas. Buenos Aires: Acmé; 1976.
- Varela RO, Brown AD. Tapires y pecaríes como dispersores de plantas en los bosques húmedos subtropicales de Argentina. In: Brown AD, Grau HR, editors. Investigación, Conservación y Desarrollo en Selvas Subtropicales de Montaña. Tucumán Argentina: Proyecto de Desarrollo Agroforestal/L.I.E.Y.; 1995. p. 129–140.
- Díaz MM Mamíferos de la Provincia de Jujuy: sistemática, distribución y ecología [ dissertation]. Tucumán Argentina: Universidad Nacional de Tucumán; 1999.
- Krebs CJ. Population cycles revisited. J Mammal. 1996;77(1):8–24.
- Myers JH. Population cycles: generalities, exceptions and remaining mysteries. Proc R Soc B. 2018;285(1875):20172841.
- Grenand P. Fruits, animals and people: hunting and fishing strategies of the Wayãpi of Amazonia. Tropical forests, people and food: biocultural interactions and applications. Vol. 13. New York: Unesco and the Parthenon Publishing group; 1993. p. 425- – 434.
- Reyna-Hurtado RA, Radachowsky J, Mcloughlin L, et al. Rapid decline of white lipped peccary populations in Mesoamerica. Proceedings of the 1st Symposium about white-lipped peccary in Mesoamerica August 25, 2016, Belize City, Belize. Belize City, Belize.; 2017.
- de Azevedo Fcc, Conforti VA. Decline of peccaries in a protected subtropical forest of Brazil: toward conservation issues. Mammalia. 2008;72:82–88.
