ABSTRACT
Nightjars (Family Caprimulgidae) are insectivorous birds with crepuscular and nocturnal habits. Their coloration and cryptic behavior make this group extremely difficult to register, being more easily heard than sighted. The rufous nightjar (Antrostomus rufus) is a widely distributed species from Southern Central America to Northern Argentina, but most aspects of its life history remain unknown. By the employment of an acoustic remote unit, we evaluated the vocal activity and environmental variables of vocalizations by rufous nightjars during a complete annual cycle at one site in the Yungas Andean Forests of Northwestern Argentina. The results, based on one sampled site, indicate that the vocal activity of this species is performed during the breeding season (between September and November). During these months, this species showed a peak of vocal activity at midnight (between 00:00 and 01:00 hs) and at dawn (06:00 hs). In addition, most vocalizations were recorded during full moon nights, and temperature was negatively related to the vocal activity. We highlight the importance of using passive acoustic monitoring as a tool for understanding the life history of nocturnal neotropical birds.
Over the last few years, progress has been made towards understanding of how both intrinsic and extrinsic factors affect nocturnal bird vocal communication. With passive acoustic monitoring (PAM), it has been possible to obtain continuous records of vocalizations during an annual period, which has increased the knowledge on the natural history of nocturnal and cryptic species, such as owls (Strigidae), pottos (Nyctibiidae), and nightjars (Caprimulgidae) [Citation1–3]. Therefore, PAM is a powerful tool for analyzing bird acoustic signals and extracting information about species presence and activity patterns [Citation4,Citation5]. This method allows obtaining continuous bioacoustics records for extended periods that are crucial for assessing the influence of biotic and abiotic environmental variables on the vocal behavior of nocturnal birds [Citation6]. For example, previous studies found a correlation between the vocal activity of an owl species from Brazil with the full moon phase and higher temperatures [Citation7]. In addition, species of neotropical potoos and nightjars are more vocally active during nights with high lunar illumination, without rain, and at the beginning of the reproductive season [Citation3,Citation5,Citation8]. Moonlight increases visibility conditions, likely causing an increase in vocal output, and in some instances, high relative humidity and temperatures can make sound transmission more efficient, while rainfall can limit the vocal activity and territorial displays during the breeding season [Citation3,Citation5].
Particularly, nightjars (Caprimulgidae) are insectivorous birds with crepuscular and nocturnal habits, distributed along a variety of environments around the world. Their coloration and behavior make them difficult to observe, being easier to hear [Citation9]. Although recent studies have provided valuable descriptions on the life history traits of caprimulgids in the Neotropics [Citation10–12] and on the vocal behavior during an annual cycle in the Brazilian Pantanal [Citation2,Citation5], there is no information on the vocalization patterns of these families in Argentina. Therefore, the use of autonomous recording units for monitoring nocturnal species might be useful for obtaining valuable life history information of the Southern cone region of South America. There are 16 species of the Caprimulgidae family in Argentina [Citation13,Citation14]. The genus Antrostomus has been recently revalidated [Citation15] with only two species breeding in the country: the rufous nightjar (A. rufus) and the dusky silky-tailed nightjar (A. sericocaudatus) [Citation16,Citation17]. Information on reproductive features of Argentinean populations (e.g. description of eggs and nestlings, breeding season) is only available for the rufous nightjar [Citation17].
The rufous nightjar is a nocturnal member of the Caprimulgidae family, with isolated populations from Southern Central America to Northern Argentina. On average, adults are 26–28 cm long with a body mass of 95 g. Male’s coloration is brown to dull rufous, whereas females are lighter in color and less rufous [Citation14]. In Argentina, the species can be found in both dry and humid forests, either in native or transformed areas. Breeding occurs between October and February, matching warm and humid seasons. During this period, females lay two eggs on the ground, which are incubated by females during the day and occasionally by males during the night [Citation17].
Based on the well-known influence of moon phases (lunar illumination) and abiotic environmental variables on this nocturnal bird species [Citation2,Citation5], we assessed the diel activity pattern vocal activity and calling phenology of rufous nightjar using an autonomous recording unit in the Yungas Andean forests of NW Argentina. Our aims were as follows: 1) identify the months and hours with the highest vocal activity during the annual cycle and 2) evaluate whether the vocal activity is influenced by the lunar phase and environmental variables (temperature, rain, and relative humidity).
The study was carried out in the Parque Nacional Calilegua, located in Jujuy Province, Argentina. The climate is characterized by a marked dry season during the winter months (July to September) and a humid season with rainfall concentrated during the spring and summer (October to March). The mean annual rainfall ranges from 800 to 1000 mm, and the average annual temperature reaches 21.1°C. Field sampling was concentrated on the lowest altitudinal stratum of Yungas, the Piedmont Forest, which constitutes a phytogeographic unit called Tropical Seasonal Forests of South America [Citation18]. We placed an Autonomous Recording Unit (ARU) SongMeter SM4 (wildlifeacoustics.com) 1.5 m above the ground (23°45’16.84“S; 64°50’59.35” W; 650 m.a.s.l), and the effective detection radius is approximately 100 m [Citation19]. The ARU remained active at the same location for one year: from September 2017 to August 2018. It was programmed to record the first 3 minutes of every hour (72 min/day), with a sampling frequency of 16 kHz, obtaining an effective recording at 8 kHz and excluding undesirable sounds. All recordings were digitized in 16-bit resolution, stored in.WAV format and then manually identified using the program Raven Pro 1.5 [Citation20]. Associated with the recorder, we installed a HOBO® MX230 data logger to register hourly temperature (ºC) and relative air humidity (RH %). Daily rain data were obtained from the Ledesma SAAI monitoring station located 7.6 km away from the study site.
All records of calling activity were analyzed using circular statistics: we estimated the mean (± SD) of the total hours recorded and then conducted a Rayleigh’s test (z) to determine if hours were uniformly distributed (p > 0.05) or showed any orientation pattern (p < 0.05) [Citation21]. We then performed a Rao’s Spacing Test (U) to explore distribution patterns with multiple modes, i.e. when multiple preferred directions (in our case hours) are expected [Citation22]. For the analysis of hours, we determined months with the highest vocal activity (September, October, and November; see ). Also, we classify the vocalizations recorded at every hour (between 20:00 and 06:00), and the moon phase during the calling activity: Full Moon, waning and waxing crescent and new Moon. We used the Chi-squared test to compare the proportion of calling activity between moon phases; as well as, to compare per every hour for each moon phase, using Infostat software (p < 0.05) [Citation23]. Moon illumination percentage data were also used for the analyses of the study (data available at the US Naval Observatory: https://aa.usno.navy.mil/data/MoonPhases).
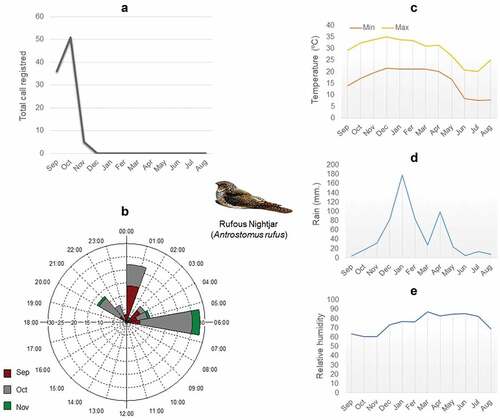
Figure 1. (a) Overall number of rufous nightjar (Antrostomus rufus) vocalizations recorded in the Piedmont Forest of Northwestern Argentina: from September 2017 to August 2018 (N = 94). (b) Circular distribution of total hours and months where vocalizations were recorded. The number of vocalizations is detailed along the axis. Besides, it includes temperature throughout the sampling year (c), as well as rainfall intensity (d), and relative humidity (e), throughout the sampling year.
Finally, to evaluate the influence of environmental variables on the vocal activity, we performed a generalized linear mixed-effects model (GLMM) with binomial distribution of errors and a Loglik link function. To balance the analysis, we randomly selected the same number of files where the rufous nightjar was not detected in the breeding season, using the sample function in R corresponding to a total of 188 sound files. All environmental variables were standardized using the standardize method [Citation24]. The response variable was presence/absence of calls (by hour) and the explanatory variables considered for the global model were temperature, relative humidity, hourly rain, and the percentage of moon phase (by hour). First, we evaluated collinearity among variables and detected that the temperature and percentage of relative air humidity were correlated (< −0.7). We then eliminated the relative humidity variable from the global model according to our previous knowledge of how this abiotic cue may relate to rufous nightjar vocal activity. We selected the best model based on the Akaike Information Criterion corrected for small samples with delta AIC c<2 [Citation24,Citation25] using the MuMin package version 1.43.17 [Citation26]. These analyses were performed on the R platform version 4.0.2 [Citation27].
We analyzed 6654 recordings (110.9 hours), 94 of which had rufous nightjar vocalizations between 20:00 and 06:00 h. We recorded no diurnal calls. Vocalizations occurred between September and November: 40% of vocalizations were recorded in September, 55% in October, and 5% in November (). The distribution of calling throughout the night was not evenly distributed (Rayleigh’s test z = 37.14, p < 0.01). There appeared to be a bimodal pattern (Rao’s Spacing test, U = 311.70, p < 0.01) (). We found significant differences in the proportion of vocalizations by nightjars depending on moon phase, being older (60%) during full moon nights (chi-square = 26.88, p < 0.01) (). Comparing between hours, we found significant differences only during new moon nights (chi-squared = 17.69, p < 0.01), with a greater proportion of calls at 06:00 a.m. Full moon and waning-waxing crescent nights, however, showed no significant differences between hours (chi-square = 12.70, p = 0.241; chi-square = 5.18, p = 0.394, respectively) (). According to the GLMM analysis, three models explain similarly the vocal activity: the model including the variable temperature was the one that best explained presence call activity in the recordings (wi = 0.44), followed by models containing moon phase (wi = 0.20) and rain (wi = 0.21) ().
Figure 2. Total percentage of vocalizations performed by the rufous nightjar (Antrostomus rufus) related to the moon phase in the Piedmont Forest of Northwestern Argentina. We detail the hours in which the vocalizations were recorded (in percentages) according to the moon phase.
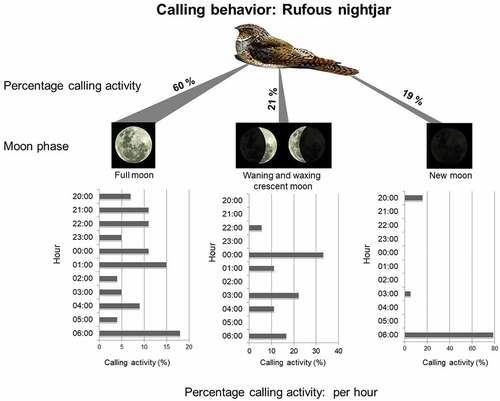
Table 1. Result of the generalized linear model assessing the relationship between the vocal activity of rufous nightjar (Antrostomus rufus) and environmental variables in the Piedmont Forest of Northwestern Argentina. AICc= Akaike’s information criterion corrected for small samples; df = degrees of freedom; wi= model probability; *full model.
The circadian calling activity pattern of rufous nightjar was characterized by two peaks, at midnight and dawn. This nocturnal bird has a marked seasonal phenology of vocalization with peaks of activity varying in intensity throughout the breeding season; as the species breeds from October to February, and vocalizations were concentrated at the beginning of the breeding season from September to November.
The moonlight could influence behavior as it is likely that males will perform reproductive displays during the brightest nights for increasing visibility [Citation2,Citation5]. Our results indicate that vocal behavior is related to the moon’s associated luminosity, because during new moon nights (lower brightness), calling activity decreased, occurring mainly at dawn; whereas on full and crescent moon nights, vocalizations are performed throughout the night. Citation3,Citation7,Citation28). In addition, the vocal activity during humid night conditions could be related to a higher foraging efficiency (vocalization for locate food), due to an increase in the availability of insects [Citation29,Citation30].
Probably, the low detection rate of the target species out of the reproductive season could be due to the restricted recording schedule employed in the present study (we recorded 3 min per hour, and only one site), compared with previous studies with nocturnal birds [Citation2, Citation5]. So that, these results should be considered as preliminary findings. Further annual monitoring studies using an array of ARUs (considering altitudinal and latitudinal gradients) are needed to increase species detection and environmental variability and identify factors associated with the vocal activity of rufous nnightjars in the Yungas Andean forests ecoregion.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- York JE, Young AJ, Radford AN. Singing in the moonlight: dawn song performance of a diurnal bird varies with lunar phase. Biol Let. 2014;10(1):20130970.
- Pérez-Granados C, Schuchmann KL. Illuminating the nightlife of two Neotropical nightjars: vocal behavior over a year and monitoring recommendations. Ethol Ecol Evol. 2020a;32(5):466–480.
- Pérez-Granados C, Schuchmann KL. Monitoring the annual vocal activity of two enigmatic nocturnal Neotropical birds: the Common Potoo (Nyctibius griseus) and the Great Potoo (Nyctibius grandis). J Ornithol. 2020b;161(4):1129–1141.
- Duchac LS, Lesmeister DB, Dugger KM, et al. Passive acoustic monitoring effectively detects Northern Spotted Owls and Barred Owls over a range of forest conditions. Condor. 2020;122(3):1–22.
- Pérez-Granados C, Schuchmann KL, Marques MI. Addicted to the moon: vocal output and diel pattern of vocal activity in two Neotropical nightjars is related to moon phase. Ethol Ecol Evol. 2021b;34:1–16.
- Sugai LSM, Silva TSF, Ribeiro JW Jr, et al. Terrestrial passive acoustic monitoring: review and perspectives. BioScience. 2019;69(1):15–25.
- Pérez-Granados C, Schuchmann KL, Marques MI. Vocal activity of the Ferruginous pygmy-owl (Glaucidium brasilianum) is strongly correlated with moon phase and nocturnal temperature. Ethol Ecol Evol. 2021a;33(1):62–72.
- Zárate V, Juncosa-Polzella AS. Vocal activity of the Great potoo (Nyctibius grandis) in relation to moonlight: detections in Costa Rica. Ornitol Neotrop. 2021;32(1):74–76.
- Holyoak DT. Nightjars and their allies: the Caprimulgiformes. Vol. 7. New York: Oxford University Press; 2001.
- Hoffmann D, Epifânio AD, de Vasconcelos MF. Nesting of Band-winged Nightjar Caprimulgus l. longirostris in eastern Brazil, including the first description of chicks. Cotinga. 2010;32:142–145.
- Pople RG. Breeding biology of the White-winged Nightjar (Eleothreptus candicans) in eastern Paraguay. Rev Bras Ornitol. 2014;22(2):219–233.
- Schaaf AA, Peralta G, Luczywo A, et al. Biologia reproductiva y comportamientos de cuidado parental de dos especies de atajacaminos de Córdoba, Argentina. Ornitol Neotrop. 2015;26:25–37.
- Bodrati A, Areta JI. Dos nuevos dormilones para la avifauna Argentina (Chordeiles acutipennis y Caprimulgus maculicaudus) y comentarios sobre hábitat, comportamiento y geonemia en Paraguay. Hornero. 2010;25(2):67–73.
- Narosky T, Yzurieta D. Aves de Argentina y Uruguay: guía de identificación. Buenos Aires, Argentina: Vasquez Mazzini Editores; 2010.
- Sigurdsson S, Cracraft J. Deciphering the diversity and history of New World nightjars (Aves: caprimulgidae) using molecular phylogenetics. Zool J Linnean Soc. 2014;170(3):506–545.
- Cleere N. Family Caprimulgidae (Nightjars). In: del Hoyo J, Elliott A Sargatal J, editors. Handbook of the birds of the world, Volume 4: barn-owls to hummingbirds. Barcelona, España: Lynx Edicions; 1999. pp. 302–386.
- Salvador SA, Bodrati A, Salvador LA. Aportes al conocimiento de la reproducción del Atajacaminos Colorado (Antrostomus rufus) en Argentina. Nuestras aves. 2014;59:54–57.
- Malizia L, Pacheco S, Blundo C, et al. Caracterización altitudinal, uso y conservación de las Yungas Subtropicales de Argentina. Ecosistemas. 2012;21(1–2):53–73.
- Boullhesen M, Vaira M, Barquez RM, et al. Evaluating the efficacy of visual encounter and automated acoustic survey methods in anuran assemblages of the Yungas Andean forests of Argentina. Ecol Indic. 2021;127:107750.
- Center for Conservation Bioacoustics. 2014. Raven Pro: interactive Sound Analysis Software. Version 1.5. Computer software. The Cornell Lab of Ornithology, Ithaca, New York. updated 2016 Nov 2 Available from: http://ravensoundsoftware.com.
- Zar, J. H. Biostatistical Analysis. Upper Saddle River, NJ: Prentice-Hall; 1999.
- Mardia KV, Jupp PE. Basic concepts and models. Mardia KV, Jupp PE. Basic concepts and models. 2nd edition ed. Chichester (UK): John Wiley and Sons; 2000. pp. 25–56.
- Di Rienzo JA, Casanoves F, Balzarini MG, et al. Software Infostat, versión 2008. Argentina: Grupo InfoStat, FCA, Universidad Nacional de Córdoba; 2008.
- Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33(2):261–304.
- Zuur A, Ieno EN, Walker N, et al. Mixed effects models and extensions in ecology with R. New York: Springer Science & Business Media; 2009.
- Barton K, Barton MK 2015. Package ‘mumin’ Version, 1,18.
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. Available from: https://www.R-project.org/
- Møller AP. When climate change affects where birds sing. Behav Ecol. 2010;22:212–217.
- Brigham RM, Barclay RM. Lunar influence on foraging and nesting activity of Common Poorwills (Phalaenoptilus nuttallii). Auk. 1992;109(2):315–320.
- Jackson HD. A review of foraging and feeding behaviour, and associated anatomical adaptations, in Afrotropical nightjars. Ostrich. 2003;74(3–4):187–204.
