Abstract
Hyponatremia due to syndrome of inappropriate antidiuretic hormone secretion (SIADH) or gastrointestinal loss of sodium is sometimes seen during chemotherapy. However, hyponatremia due to renal salt wasting syndrome (RSWS) can rarely occur during cisplatin-containing chemotherapy. We report a case of a 65-year-old man with recurrent oropharynx cancer treated with cisplatin, docetaxel and 5-fluorouracil-containing chemotherapy who suffered from acute-onset consciousness disturbance due to hyponatremia just after administration of cisplatin. His serum sodium level was 109 mmol/L, urinary sodium was 132 mmol/L, and the urinary output in 6 h was 3700 mL. In other words, he had hyponatremia, hypernatriuria and the excessive urinary excretion of sodium; the diagnosis of RSWS was made. The patient was treated with supplement of sodium and isotonic solution with careful monitoring of serum sodium level. He responded to the treatment well and recovered from the hyponatremia episode in a few days without any complications. Because treatment for hyponatremia caused by SIADH or RSWS was totally different, i.e. water depletion for SIADH and sodium supplies for RSWS, it is important to make a correct diagnosis for hyponatremia seen during chemotherapy. In this report, we present a precise clinical data of RSWS patients and also summarize the key diagnostic features of RSWS which can help to differentiate from other hyponatremia syndromes.
Introduction
Hyponatremia is a well-described complication of chemotherapy, and a variety of situations can be of its origin including syndrome of inappropriate antidiuretic hormone secretion (SIADH), gastrointestinal losses, diuretic use, osmotic diuresis, inadequate supplies, cardiac, renal, or hepatic failure.[Citation1,Citation2] Renal impairment is an also well-known adverse effect of chemotherapy caused by nephrotoxicity due to a decrease of glomerular filtration, leading to an elevation of blood creatinine and blood urea nitrogen (BUN). However, chemotherapy-induced damages of proximal tubes could cause hyponatremia associated with volume depletion, resulting in natriuresis with an increase in urine output and urine sodium known as renal salt wasting syndrome (RSWS).[Citation3] Although RSWS is a rare condition during chemotherapy, treatment for hyponatremia caused by SIADH and RSWS was totally different, i.e. water depletion for SIADH or sodium supply for RSWS. Therefore, in order to perform an appropriate treatment for hyponatremia, correct diagnosis is necessary. Herein we present a patient with severe hyponatremia during chemotherapy for recurrent oropharyngeal cancer caused by RSWS. We also summarize the key diagnostic features of RSWS which can help to differentiate it from other hyponatremia syndromes.
Case report
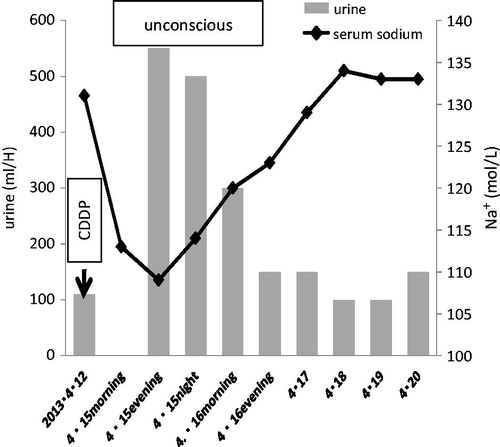
A 65-year-old man was admitted to Osaka National Hospital with diagnosis of oropharynx cancer (right lateral wall, T3N1M0) in October 2007. As a primary treatment, the patient underwent a neoadjuvant chemotherapy, consisting of cisplatin, 5-fluorouracil and docetaxel for one cycle followed by right lateral pharyngotomy with modified radical neck dissection. After these treatments, he received radiotherapy (60 Gy/30 fr.). In the next five years, loco-regional recurrence was seen three times. He underwent operation three times and chemotherapy for seven cycles, consisting of cisplatin, 5-fluorouracil and docetaxel as well. In February 2013, he was admitted to the above hospital due to local recurrence that was not resectable due to carotid invasion. Vital signs were within normal range. Laboratory tests showed a hemoglobin level of 11.7 g/dL, serum sodium of 137 mmol/L, blood urea nitrogen/creatinine ratio (BUN/Cr) of 27.2, and a normal glomerular filtration rate (67 mL/min). Chemotherapy with cisplatin, 5-fluorouracil and docetaxel was performed, i.e., a continuous infusion of docetaxel 48 mg/m2/day on day 1, 5-fluorouracil 600 mg/m2/day on days 1–5, cisplatin 48 mg/m2/day on day 4. Adequate hydration was ensured by administration of 1–2 L fluid per day for 3 days prior to cisplatin administration to prevent nephrotoxicity. Neither during nor after this cycle, did he not have any complaint of polyuria, lightheadedness, vomiting nor diarrhea. At that time, his serum sodium was 132 mmol/L, and estimated glomerular filtration rate was 52 mL/min. Thirty-six days later, the next identical cycle of chemotherapy. At the 4th day of this cycle, he developed convulsions accompanied by unconsciousness after severe malaise. Laboratory studies showed a sodium level of 109 mmol/L, potassium level of 2.6 mmol/L, magnesium level of 0.9 mmol/L, BUN/Cr ratio of 35.6, GFR of 104 mL/min/1.39m2, urine sodium of 132 mmol/L (40–90 mmol/L), urine osmolality of 468 mOsm/L (50–1300 mOsm/L), serum osmolality of 230 mOsm/L (270–295 mOsm/L), serum cortisol of 43.5 μg/dL (4–19.3 μg/dL), fractional excretion of sodium of 3.1% (FENa), urine β2-microglobulin of 3.93 mg/L (β2MG: 0–0.2 mg/L), and normal thyroid stimulation hormone level. Antidiuretic hormone could not be measured due to mechanical trouble. Computed tomography of the brain did not show any lesion. In addition, polyuria (3700 mL/6 h) was also noted. It was supposed that increased urinary sodium excretion was due to damages of proximal tubules induced hypernatriuria and polyuria, resulting in acute symptomatic hyponatremia. Since he was in a coma from hyponatremia and had dehydration from polyuria, a treatment had to be immediately started: restoring the intravascular volume and correcting electrolyte imbalance with careful monitoring of serum and urinary electrolytes and water balance at every 2 h. His consciousness gradually improved with an increase in his serum sodium. After 3 days of onset, his serum sodium recovered to 134 mmol/L (Figure ). Regarding the differential diagnosis of hyponatremia, diabetes insipidus could be excluded because he had hypernatriuria instead of hyponatriuria, syndrome of inappropriate antidiuretic hormone (SIADH) was not likely because he was dehydrated. High FENa and high urine β2MG strongly suggested tubular damages perhaps induced by cisplatin. Finally, he was diagnosed as having renal salt wasting syndrome (RSWS). One month later, he was discharged from the hospital, however, oral sodium intake was necessary to keep appropriate serum sodium level.
Discussion
Hyponatremia is a common potential complication in cancer patients. The incidence of hyponatremia in a hospitalized population varies greatly according to the type of patients studied and to the definition of hyponatremia. These incidence rates range from less than 1% to more than 40%.[Citation1] A variety of situations can be the origin of a hyponatremia in a cancer patient; anticancer medical therapy, diuretic use, gastrointestinal loss, hypotonic infusions, SIADH, cardiac, renal or hepatic failure and other causes.[Citation4,Citation5] Vinca alkaloids, platinum compounds, alkylating agents, and immunomodulators are reported as anticancer drugs which can induce hyponatremia. As regards platinum, cisplatin is more frequently described than carboplatin.[Citation1] The incidence of hyponatremia secondary to cisplatin can be as high as 43%,[Citation6] the mechanism of action by which cisplatin induces hyponatremia is controversial. There are two possibilities: RSWS and SIADH.[Citation7–9]
Cisplatin is believed to exert its damage on the different segments of the nephron. A well-known elevation of BUN and serum creatinine is induced by a decrease of glomerular filtration rate. RSWS occurs when cisplatin damages the proximal tubules, the major site of sodium and water re-absorption, leading to an obligatory polynatriuresis, hyponatremia and hypovolemia, with an increase in urine output and urine sodium. Thus, the treatment of RSWS aims at restoration of euvolemia through water and salt administration.[Citation3]
On the other hand, many conditions have been associated with SIADH, which may be classified into four major groups including neoplasia, neurological disorders, lung disease and an increasing variety of drugs, such as vinca alkaloids, cisplatin and chlorpropamide.[Citation10,Citation11] The mechanism of cisplatin by which SIADH occurs is unclear.[Citation1]
The diagnosis of SIADH should be considered if the five cardinal criteria are fulfilled (hypotonic hyponatremia, natriuresis, urine osmolality in excess of plasma osmolality, absence of edema and volume depletion, normal renal and adrenal function). The treatment of SIADH is fluid restriction, or only for significantly symptomatic patients or those with symptomatic acute hyponatremia, hypertonic (3%) or even isotonic saline infusion should be used.[Citation8] We summarize the major differential features for RSWS and SIADH in Table .
Table 1. Differential diagnostic features and treatment for SIADH and RSWS.
In summary, cisplatin is one of the most widely used anticancer drugs. Although its nephrotoxicity, renal impairment with a decline in glomerular filtration, is well known, RSWS is not common so far. Oncologists have to know the existence of cisplatin-induced hyponatremia due to RSWS as well as that led by SIADH. Treatment strategy for hyponatremia is totally different between these two pathophysiologies.
Acknowledgements
We thank Dr. Ryohei Oya and Dr. Yujin Yamamura for the support. Dr. M. Inamori takes responsibility for the integrity of the content of the paper. The research was conducted in Department of Otolaryngology, Osaka National Hospital.
Funding
This work was not supported by any agency grant.
References
- Berghmans T. Hyponatremia related to medical anticancer treatment. Support Care Cancer. 1996;4:341–350.
- Matsuura T. Hyponatremia in cancer patients. Jpn J Nephrol. 2012;54:1016–1022.
- Hamdi T, Latta S, Jallad B, et al. Cisplatin-induced renal salt wasting syndrome. South Med J. 2010;103:793–799.
- Berghmans T, Paesmans M, Body JJ. A prospective study on hyponatremia in medical cancer patients: epidemiology, aetiology and differential diagnosis. Support Care Cancer. 1999;8:192–197.
- Yajima Y, Fujii M, Tokumaru Y, et al. Hyponatremia with cisplatin administration in head and neck cancer patients. Jpn J Cancer Chemother. 2010;37:2861–2865.
- Lee YK, Shin DM. Renal salt wasting in patients treated with high-dose cisplatin, etoposide, and mitomycin in patients with advanced non-small cell lung cancer. Korean J Intern Med. 1992;7:118–121.
- Vanhees SL, Paridaens R, Vansteenkiste JF. Syndrome of inappropriate antidiuretic hormone associated with chemotherapy-induced tumor lysis in small-cell lung cancer. Ann Oncol. 2000;11:1061–1065.
- Nomura M, Kamata M, Kojima H, et al. Renal salt-wasting syndrome associated with docetaxel in an esophageal cancer patient. Int Canc Conf J. 2012;1:67–69.
- Cheng CY, Lin YC, Chen JS, et al. Cisplatin-induced acute hyponatremia leading to a seizure and coma. Chang Gung Med J. 2011;34:48–51.
- Baylis PH. The syndrome of inappropriate antidiuretic hormone secretion. Int J Biochem Cell Biol. 2003;35:1495–1499.
- Ellison DH, Berl T. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356:2064–2072.