Abstract
The aim of the authors is to document positional alcohol nystagmus type I (PAN I) elicited by positioning in pitch plane in order to activate/inhibit the vertical canals. The second aim of the present paper is to review possible nystagmus patterns in benign paroxysmal positional vertigo (BPPV) by creating a comprehensive framework for peripheral positional vertigo. Beside paroxysmal variants, also more subtle, persistent nystagmus types have to be integrated into this framework; therefore the use of the term ‘peripheral positional vertigo and dizziness’ (PPVD) is suggested. In four healthy individuals the first phase of the PAN I was studied minimum 60 min after alcohol ingestion. As anticipated, positioning from sitting into head hanging prone position elicited persistent purely upbeat nystagmus, and then the positioning back from this position evoked a purely downbeat nystagmus with similar dynamics. These results help to understand the different types of PPVD. It is possible to elicit purely vertical upbeat and downbeat nystagmus by symmetrical positioning in head hanging prone, head hanging supine and sitting positions after alcohol ingestion (PAN 1).
Introduction
The archetype of benign paroxysmal positional vertigo (BPPV), the most frequent, most conspicuous variant is the canalolithiasis of the posterior canal in which, according to the accepted theory, freely moving otoconial debris are trapped in long arm of the posterior semicircular canal.[Citation1] This sensitizes the semicircular canal (SCC) to changes of the gravity vector. Another paroxysmal variant is the canalolithiasis of the horizontal canal, which is also well documented.[Citation2] However, more subtle variants also exist, in which the nystagmus is persistent instead of being paroxysmal. Present authors encountered clinical cases in which similar nystagmus could be evoked in the Dix–Hallpike position to that seen in typical canalolithiasis but instead of being crescendo–decrescendo, it was slight, persistent and of long duration. This nystagmus could hypothetically be explained by a light cupula of either the anterior or the posterior vertical SCC. Since these more subtle, persistent nystagmus types have to be integrated into a comprehensive framework of all peripheral positional vertigo variants we find it more appropriate to use the term ‘peripheral positional vertigo and dizziness’ (PPVD). These latter persistent nystagmus types may be caused by otoconia, which by adhering to the cupula make it sensitive to gravity or by pathological processes when the cupula itself attains a specific density differing from that of the endolymph. In the last few years it became clear that in certain pathological conditions (which may be associated with migraine [Citation3]) the cupula may become lighter than the surrounding endolymph: the ‘light cupula’. The light cupula syndrome of the horizontal canal has been well documented in patients [Citation4] and has also been characterized in model experiments using density changes after alcohol ingestion.[Citation5] Concerning the possibility of a light cupula in the vertical canals, the present authors have their own clinical observations but found no publications in the literature. There is only one publication [Citation6] concerning vertical nystagmus during the alcohol influence on the vestibular system. After alcohol ingestion the authors positioned the subjects such that the lateral canals were earth vertical and then rotated them in the plane of the lateral canals about an earth-horizontal axis to either 45° or 90°, right or left ear down. The spatial analysis of the responses showed that in addition to the nystagmus induced by the buoyancy of all six cupulae, alcohol intoxication also caused a vertical velocity offset (in all subjects, slow phase down) that is independent of the orientation of the subject in space. It was suggested that this bias is caused by a toxic effect on central vestibular pathways. However, in our view, using this experimental paradigm (lateral supine position) it is very difficult to determine the exact position of the vertical canal ampullae. Therefore, in the present examinations our aim is to document positional alcohol nystagmus type I (PAN I) elicited by symmetrical positioning in head-hanging prone, head hanging supine and sitting positions in order to activate/inhibit the vertical canals. This is apparently possible and the elicited nystagmus types correspond to the anticipated eye movements if the three-dimensional positions of the anterior and posterior canal cupulae, the starting position and the final, provoking position are taken into account. The second aim of the present paper is to review possible nystagmus types in BPPV. Here it is suggested that it is possible to explain all the variants seen in BPPV, such as the direction-changing horizontal nystagmus in lateral supine position, other subtle and persistent types as the peripheral downbeat nystagmus or BPPV without any nystagmus. One only has to bear in mind that otoconial debris may float into any canal, into its short arm or long arm, be attached onto any cupula and that sometimes in absence of free floating otoconia, cupular pathology may cause the symptoms, as in the ‘light cupula’ disorders. After defining the possible nystagmus directions, their temporal and intensity patterns and the necessary examination methods, a comprehensive classification of possible BPPV variants will be attempted and the underlying hypothetical pathologies will be described.
Methods
Subjects
Four male individuals (30, 42, 52 and 56 years old) took part in the experiment. To elicit PAN, the participants drank 0.68 g ethanol per kg body weight during 15 min, diluted about 1:3 in a soft drink. The presence of ethanol on the breath was control by an alcohol analyzer (Safeway, Paramint AB, Uppsala, Sweden) with a detection range from 0.1% to 1.0%.[Citation5]
The subjects gave their consent, although they were free to withdraw at any time during the experiment. The test procedure was in accordance with the Declaration of Helsinki and was approved by the Human Ethics Committee at the hospital.
Head positions
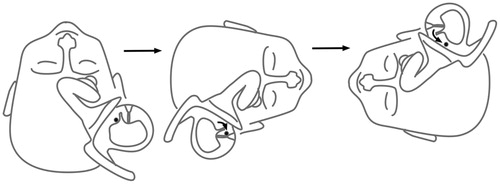
Before alcohol ingestion, nystagmus was measured during a series of head positions in pitch plane in the following order: sitting (Reid’s base line in horizontal position), head hanging prone position (head bent forward, Reid’s base line in vertical position), again sitting position, supine position with the head elevated 30° (Reid’s base line in vertical position) then again sitting position, head hanging supine position (like in the Dix–Hallpike position without turning head to the side) and then again sitting position.
Then, after having ingested the alcohol, 60 min later, the existence of PAN I was verified by eliciting the well-documented persistent, slight, direction-changing, geotropic horizontal nystagmus evoked by left and right lateral supine position.[Citation5] Then, during the phase of PAN I the above positioning protocol was repeated and the provoked nystagmus recorded (as shown in Figure ).
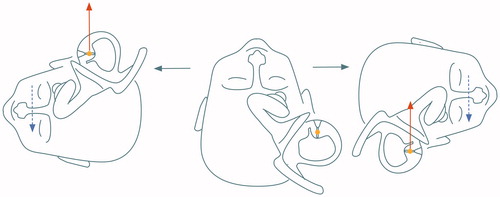
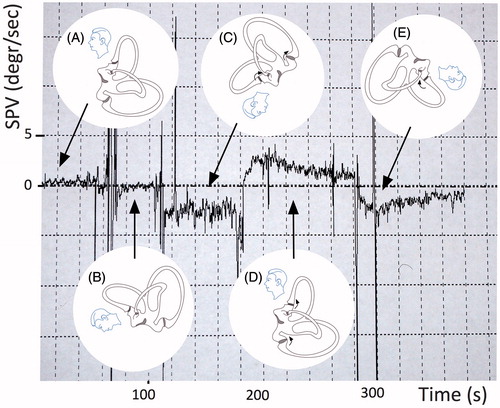
Figure 1. Example of vertical slight, persistent positional nystagmus evoked by symmetrical positioning during PAN I. (A) Sitting position. This position has been maintained for a longer time before the measurements therefore the vertical semicircular cupulae are already deflected upward and adapted to this situation. (B) From sitting to 30° prone (no nystagmus because the position of the vertical cupulae does not change), (C) from 30° prone to head-hanging prone (upbeat nystagmus because of the deflection of the anterior and posterior cupulae (curved arrows)), (D) from head-hanging prone to sitting (downbeat nystagmus for over 100 s), and (E) from sitting to supine: again upbeat nystagmus (because of the deflection of the anterior canal cupula).
Nystagmus recording
Two instruments for video-nystagmography (SMI C-A Tegnér AB, Stockholm, Sweden, and 3D Ulmer VNG, Torsio Synapsys, France) were used simultaneously for visualization of eye movements and graphical recording of nystagmus. Nystagmus was digitally stored for quantification of nystagmus slow phase velocity (SPV), which was registered in the vertical and horizontal directions.
In the different positions, nystagmus was considered to be present when five or more consecutive nystagmus beats were identified. The period of 10 s with the highest mean velocity was regarded as representative for the position. Since the deviation of the cupula depends on the starting versus provoking position, nystagmus was analysed in position pairs.
Sitting, sitting to prone, from prone to forward head-hanging prone, from head-hanging prone to sitting, from sitting to supine and from sitting to head-hanging supine.
Spontaneous nystagmus was defined as nystagmus occurring when the head was kept upright and straight, forward in a position normal for the test subject.
Results
Alcohol level
For all four subjects the breath ethanol level ranged from 0.4 to 1.0 promille (maximum detection level for analyzer) during the first 80 min of the trial. The concentrations are considered sufficient to elicit PAN I.[Citation7]
Nystagmus directions during positioning
At the beginning of the PAN I the horizontal geotropic PAN I could be elicited in both lateral supine positions. This nystagmus, which was expected and studied earlier signalized the beginning of PAN I.[Citation7] In all cases it was possible to document the vertical component of alcohol nystagmus elicited by symmetrical, straight prone and supine positioning in the pitch plane. The torsional component in all cases was negligible. In sitting, before positioning there was no nystagmus. When the subject was positioned from sitting into straight head hanging prone position, according to our expectations a persistent, reproducible upbeat nystagmus occurred. When the subject was returned into sitting position, a similar but downbeat nystagmus could be evoked. This vertical PAN I lasted in all cases longer than 1 min. Vertical persistent PAN evoked by different head positions in pitch plane is shown in Figure .
The data concerning the vertical nystagmus for four subjects are summarized in Table .
Table 1. Direction and intensity of the vertical nystagmus elicited by symmetrical positioning (around the y-axis with pitch rotation).
Discussion
Although horizontal light cupula in pathological clinical cases and after alcohol is well documented, there are only a few data concerning the behaviour of the vertical canal cupulae in the first phase of PAN. Fetter et al.[Citation6] documented PAN I vertical nystagmus which was elicited by positioning into supine position and then turning the person to the right and left. According to the authors alcohol intoxication also caused a vertical velocity offset that was thought to be independent of the orientation of the subject in space. This was contributed to the toxic effect of alcohol on central vestibular pathways. However, in lateral supine position it is very difficult to ascertain the exact position of the individual vertical cupulae. Therefore the aim of the present authors was to carry out symmetrical positioning around the pitch axis in order to elicit vertical canal inhibition/stimulation. When planning the experiments and analysing data, it is very important to record the starting position as well as the new position, because if a certain head position is maintained, the afferent activity may decline with adaptation even if the cupula is continually displaced.[Citation8] This is to be seen in Figure between ‘A’ position and ‘D’ position. Although these positions are the same (sitting), in ‘A’ there is no nystagmus, because the person has been sitting for before and the cupulae (although buoyed) adapted. Then after in the head hanging prone position the cupulae deviate into the other direction, and the adaptation begins. This explains the strong nystagmus reaction when the examined person reaches the sitting position for the second time. It is always the change of the cupula position, which elicits nystagmus.
According to these results it is possible to systematically vary the spontaneous rate of the vertical cupulae shortly after alcohol ingestion, during the first phase of PAN. These results lack the necessary statistical numbers to decide if there is a central bias of vertical nystagmus after alcohol. To decide this, more examinations are planned.
It is well known, that gravity may enhance a pre-existing spontaneous downbeat nystagmus via the otolithic pathways when the patient’s head is upside-down or in prone or supine position and that downbeat nystagmus may occur in healthy subjects with an upside-down position of the head.[Citation9] We are confident that the vertical nystagmus, which we encountered after alcohol ingestion was truly peripheral because there were no nystagmus beats in the same positions during the control measurements before alcohol and because of the good reproducibility of the nystagmus reversal after each positioning. Another argument is that the instensity of vertical nystagmus in the present study is higher than the values considered as normal variation of vertical nystagmus in positional testing.[Citation10] According to the literature [Citation5,Citation6] alcohol acts on all functioning cupulae simultaneously. Therefore, the alcohol nystagmus seen during symmetrical positioning in prone and sitting position, as done in our study, is purely vertical because the torsional components originating in the right and left anterior and right and left posterior SCCs cancel each other out and the purely vertical component remains.
Clinically, slight, persistent, geotropic-upbeat nystagmus (similar to that seen in posterior canalolithiasis, but without the typical crescendo character and of longer duration) may be caused by posterior light cupula, such as seen in cases with partial isolated inferior vestibular nerve involvement in vestibular neuritis. These cases are typically resistant to reposition manoeuvres (such as the Epley-manoeuver). Our observations may sensitise clinicians in observing these clinical patterns.
Framework of PPVD – review of the literature
Dynamics of canalolithiasis and cupulopathies
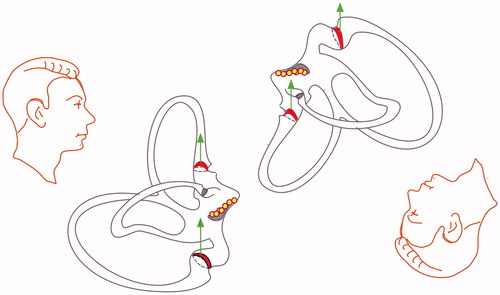
In canalolithiasis debris moves around in the long arm of the canal or in the short arm, between the cupula and utricle (Figure ). During their movement, debris may also attach to any cupula on either side (cupulolithiasis). According to physics, debris attached to the cupula has to be relatively small, because in case of big particles their weight would exceed the adhesive forces (volume, therefore mass is scaling by a factor of 3, compared to radius). Although adhesion decreases with increasing particle size, bigger particles are easier to remove from an adhesive surface.[Citation11] Concerning the dynamics of the elicited nystagmus in cupulo versus canalolithiasis, Otsuka et al.[Citation12] demonstrated in model experiment on bullfrog labyrinths that canalolithiasis is characterized by an intensive, transient crescendo–decrescendo nystagmus with a latency of several seconds and a short duration. In a model experiment using bullfrog labyrinth, cupulolithiasis presented with negligible latency and longer duration (more than several minutes). The authors explained the latency in canalolithiasis by the otoconial movements and their frictional interaction with the canal wall: at the beginning the otoconia moved slowly and then accelerated. Other models and experiments confirmed these findings.[Citation13]
Light cupula
In the last few years a new concept evolved when authors wanted to explain the presence of a horizontal persistent geotropic nystagmus similar to that seen in PAN phase I.[Citation4] The theory behind PAN I has been proposed in publications by Aschan et al.[Citation7] and Oosterveld.[Citation14] According to the buoyancy theory, the cupula, due to high concentration of alcohol, has become lighter than the surrounding endolymph.[Citation7] The dynamics of this nystagmus type correspond to that of seen in patients with persistent horizontal geotropic nystagmus. Therefore the hypothesis of ‘light cupula’ of the horizontal canals has been put forward (see Figure ). Furthermore, clinical tests have been carried out to mimic this syndrome. It was found that a persistent geotropic nystagmus could be elicited in subjects with only one SCC functioning (unilateral vestibular loss) after ingestion of alcohol.[Citation15] However, in a study of 20 consecutive patents with a persistent geotropic nystagmus, the authors failed to find a nystagmus pattern that would allow a lateralization of the affected side.[Citation3]
In cases of a horizontal canalolithiasis, the nystagmus is geotropic and stronger when the affected ear is lowermost as the cupula deviates towards the utricle. This is because of Ewald’s second law, which states that in the horizontal canal there is a stronger nystagmus response, when the endolymph in the long arm of the SCC exerts pressure towards the cupula than away from the cupula. However, in the study, referred to above, by Tomanovic and Bergenius [Citation3] the geotropic nystagmus in large majority of the patients was of low intensity and there was no difference in nystagmus velocity between the two directions. This may indicate that the cupula deviation in these patients was too small to show the intensity differences according to Ewald’s second law. In theory, it should also be possible to lateralize a light cupula of the lateral SCC according to, the so called ‘zero’ or ‘neutral’ positions of the head, when no nystagmus can be elicited because the long axis of the cupula is aligned with the gravitational vertical. Several authors recommended the zero position for determining the involved side.[Citation16] This should be possible, if the exact normal anatomic position of the cupula was known. To date, the topographical orientation of the long axis of the cupula remains disputed. In three-dimensional reconstructions the axis of the cupula seems to be closely parallel to the median line of the head.[Citation17] It has also been noted that the neutral position varies greatly. There is one more effect, which makes it difficult to search for a neutral position. If a head position is maintained, then, after a while, the semicircular afferent activity attenuates with adaptation, even if the cupula is deflected permanently. This adaptation makes it difficult to decide if the nystagmus disappeared because the attenuation or because of an eventual neutral position.
Tomanovic and Bergenius argued that small deviations in either direction from the median line could be within normal anatomic variations and that pathological processes may cause alterations of the precise position of the cupula. For example, increased fluid volume within the endolymphatic system could alter the orientation of the cupular axis and shift the orientation of the axis from the median line. Therefore they did not recommend to determine the involved side using nystagmus direction according to the neutral or zero position.[Citation3]
Nystagmus types in PPVD
In the last few years, attempts have been made to describe and explain new, less frequent nystagmus patterns in peripheral positional vertigo, other than in the already well-accepted posterior canalolithiasis and horizontal canalo/cupulolithiasis. We present in Table , a hypothetical topodiagnostic classification which originates in the anatomy of the vestibular labyrinth.[Citation1,Citation18–22]
Table 2. Possible nystagmus types in peripheral positional vertigo and dizziness – a hypothetical topodiagnostic classification based on the pathophysiology of the vestibular labyrinth (the nystagmus in posterior canalolithiasis is called upbeat-geotropic, because in the Dix–Hallpike position the patient’s head is hanging upside down and an upbeat nystagmus with a fast component toward the centre of the earth also beats towards the top of the head. On the other hand, nystagmus beating down, in the direction of the chin, such in anterior canalolithiasis, is called positional downbeat nystagmus; although in head-hanging position, this is in fact apogeotropic. The torsional component is named by the fast movement of the upper pole of the eyes).
Some of these patterns are paroxysmal, such as the paradoxically direction-changing horizontal nystagmus in lateral supine position, others subtle and persistent as the peripheral downbeat nystagmus. Apart from the nystagmus types provoked by head-position changes, theories have been put forward to explain BPPV even without any nystagmus provocation at all. It has been even suggested that this variant may be more frequent that anticipated. Present classification for BPPV is based on hypothetical behaviour of detached otoliths and their localisation in different parts of the SCCs. We suggest a classification based on nystagmus characteristics. Using such a classification, based on symptoms, makes it possible to avoid speculations concerning putative pathophysiological mechanisms.
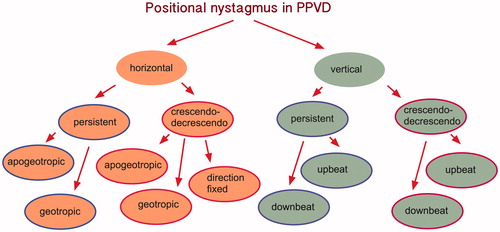
Below is a new classification, based on the dynamics of the nystagmus (persistent vs. crescendo, decrescendo) and on the plane of the provoked nystagmus (vertical vs. horizontal) (Figure ).
Figure 4. New classification is suggested, based on the dynamics of the nystagmus (persistent vs. crescendo, decrescendo) and on the plane of the provoked nystagmus (vertical vs. horizontal).
Positional nystagmus in the horizontal plane
This is provoked by the patient in the supine position with head elevated 30° from horizontal plane carrying out head rolls to both lateral positions.
Persistent horizontal positional nystagmus. Long lasting and persistent apogeotropic horizontal nystagmus (beating laterally up in both lateral supine positions) stronger on the contralateral side, when the involved ear is uppermost: lateral canal heavy cupula (cupulolithiasis).
Long lasting and persistent geotropic horizontal nystagmus (beating laterally down in both lateral supine positions): lateral canal light cupula. Lateralization is not possible according to nystagmus intensity.
Crescendo–decrescendo horizontal positional nystagmus. Crescendo–decrescendo apogeotropic horizontal nystagmus beating laterally up in unilateral supine position only when the involved ear is lowermost: lateral canal short arm canalolithiasis (otoconial debris falls onto the cupula during head-turning).
Crescendo–decrescendo geotropic horizontal nystagmus beating laterally down in both lateral supine positions, stronger on the ipsilateral side, when the involved ear is lowermost: lateral canal canalolithiasis.
Direction fixed nystagmus and unilateral apogeotropic horizontal crescendo–decrescendo nystagmus can be caused by short arm canalolithiasis and other atypical canalolithiasis.[Citation21]
Positional nystagmus in the vertical plane
This is provoked by the so-called Dix–Hallpike positioning.
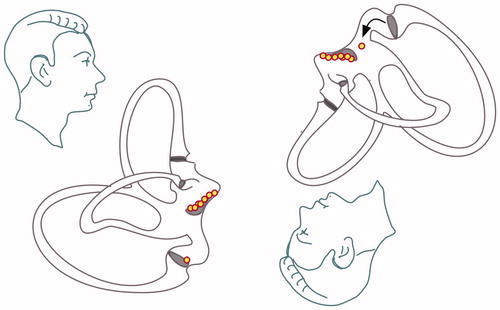
Persistent vertical positional nystagmus. Long lasting and persistent downbeat-torsional nystagmus provoked in the Dix–Hallpike position on both sides: cupulolithiasis (heavy cupula) of the posterior canal or of the contralateral anterior canal (lateralization is not possible, since provoked bilaterally) (see Figure ).
Figure 5. Posterior cupulolithiasis: in cases of posterior cupulolithiasis, depending on the precise position of the inferior ampulla, either no nystagmus or a slow downbeat nystagmus should ensue when the patient is positioned from sitting to the Dix–Hallpike position. This downbeat nystagmus of frequently seen after a successful Epley manoeuver because the debris is moving into the utriculus and is deposited in its most inferior part, which is the posterior ampulla.
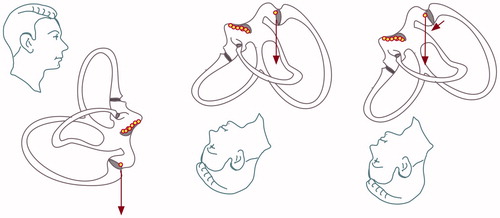
Long lasting and persistent upbeat-torsional nystagmus provoked in the Dix–Hallpike position on both sides: light cupula of the posterior canal or of the contralateral anterior canal (lateralization is not possible, since provoked bilaterally) (see Figure ).
Crescendo–decrescendo vertical positional nystagmus. Crescendo–decrescendo upbeat-torsional nystagmus with a small torsional fast-phase, which beats toward the affected ear (affected ear lowermost in Dix–Hallpike position): long arm canalolithiasis of the posterior canal.
Crescendo–decrescendo downbeat nystagmus with a small torsional fast-phase, which beats toward the affected ear (affected ear uppermost in Dix–Hallpike position): long arm canalolithiasis of the anterior canal
No nystagmus in Dix–Hallpike position
No nystagmus in the Dix–Hallpike position but sitting up vertigo either in the RALP or LARP plane: possibly posterior cupulolithiasis or posterior short-arm canalolithiasis (see Figure ), if central vestibular causes are excluded (so called ‘type 2 BPV’ [Citation23]).
In conclusion we argue that, although mainly the classical canalolithiasis has immediate practical relevance since they make an effective therapy (reposition manoeuvre) possible, nevertheless it is important to know all possible nystagmus types in order to make the differential diagnosis. The theories and hypothetical mechanisms presented here may help clinicians in their effort in diagnosing BPV. Further work is necessary to verify the above hypotheses. At the dawn of neurotology the strongest, most paroxysmal nystagmus types defined positional vertigo.
Nowadays it is possible to record and document more subtle, slight, persistent nystagmus types and also the hypothetical framework evolved, these low intensity, persistent nystagmus types (and, as we see above, perhaps positional vertigo even entirely without provoked nystagmus) have to be integrated into the framework of all peripheral positional vertigo variants. Therefore we recommend using the term ‘peripheral positional vertigo and dizziness’. We define this by chronic symptoms and complaints characteristic of positional vertigo (dizziness when bending forward, looking up, or turning over in bed) and one of the nystagmus types characterized above. In clinical settings, it is therefore recommended that activation/inhibition should be documented by additionally testing the patients in lateral supine, lateral prone and straight prone positions and to record both horizontal and vertical nystagmus velocity/duration of nystagmus at least for 100 s.
We stress that, since central vestibular pathology may also cause these symptoms,[Citation24] PPVD should always be an exclusion diagnosis, when more dangerous entities have been excluded.
Notes on contributors
Dr. Tatjana Tomanovic, planned the experiments, carried out the measurements, data analysis and wrote the manuscript. She has been working as clinician, research scientist and teacher at the Department of Audiology and Neurotology, Karolinska University Hospital since 2002. This study is further development of the light cupula concept (dr TT thesis).
Dr. Buki Bela, planned the experiment, carried out data analysis and wrote the manuscript. He has been working at the ENT Department of the County Hospital in Krems since 1998. Since two years ago the institution become the Otolaryngology Clinic of the Karl Landsteiner Medical University. Dr. BB has been participating in teaching and research.
Acknowledgements
The authors thank Docent Johan Bergenius for his stimulating suggestions.
References
- Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). CMAJ. 2003;169:681–693.
- McClure JA. Horizontal canal BPV. J Otolaryngol. 1985;14:30–35.
- Tomanovic T, Bergenius J. Vestibular findings in patients with persistent geotropic positional nystagmus: the ‘light cupula’ phenomenon. Acta Otolaryngol. 2014;134:904–914.
- Bergenius J, Tomanovic T. Persistent geotropic nystagmus – a different kind of cupular pathology and its localizing signs. Acta Otolaryngol. 2006;126:698–704.
- Tomanovic T, Bergenius J. Can the nystagmus pattern in patients with a ‘light cupula' be reproduced in hemi-labyrinthectomized subjects during positional alcohol nystagmus 1? Acta Otolaryngol. 2011;131:929–936.
- Fetter M, Haslwanter T, Bork M, et al. New insights into positional alcohol nystagmus using three-dimensional eye-movement analysis. Ann Neurol. 1999;45:216–223.
- Aschan G, Bergstedt M. Positional alcoholic nystagmus (PAN) in man following repeated alcohol doses. Acta Otolaryngol Suppl. 1975;330:15–29.
- Rabbitt RD, Breneman KD, King C, et al. Dynamic displacement of normal and detached semicircular canal cupula. J Assoc Res Otolaryngol. 2009;10:497–509.
- Pierrot-Deseilligny C, Milea D. Vertical nystagmus: clinical facts and hypotheses. Brain. 2005;128:1237–1246.
- Martens C, Goplen FK, Nordfalk KF, et al. Prevalence and characteristics of positional nystagmus in normal subjects. Otolaryngol Head Neck Surg. 2016;154:861–867.
- Corn M. The adhesion of solid particles to solid surfaces. I. A review. J Air Pollut Control Assoc. 1961;11:523–528.
- Otsuka K, Suzuki M, Furuya M. Model experiment of benign paroxysmal positional vertigo mechanism using the whole membranous labyrinth. Acta Otolaryngol. 2003;123:515–518.
- Rajguru SM, Rabbitt RD. Afferent responses during experimentally induced semicircular canalithiasis. J Neurophysiol. 2007;97:2355–2363.
- Oosterveld WJ. Effect of gravity on positional alcohol nystagmus (PAN). Aerosp Med. 1970;41:557–560.
- Tomanovic T, Bergenius J. Is the nystagmus pattern in hemi-labyrinthectomized subjects during positional alcohol nystagmus 2 similar to that found in patients with cupulolithiasis in the lateral semicircular canal? Acta Otolaryngol. 2013;133:796–803.
- Kim CH, Shin JE, Kim YW. A new method for evaluating lateral semicircular canal cupulopathy. Laryngoscope. 2015;125:1921–1925.
- Curthoys IS, Uzun-Coruhlu H, Wong CC, et al. The configuration and attachment of the utricular and saccular maculae to the temporal bone. New evidence from microtomography-CT studies of the membranous labyrinth. Ann N Y Acad Sci. 2009;1164:13–18.
- Aw ST, Todd MJ, Aw GE, et al. Benign positional nystagmus: a study of its three-dimensional spatio-temporal characteristics. Neurology. 2005;64:1897–1905.
- Vannucchi P, Pecci R, Giannoni B. Posterior semicircular canal benign paroxysmal positional vertigo presenting with torsional downbeating nystagmus: an apogeotropic variant. Int J Otolaryngol. 2012;2012:413603.
- Buki B. Benign paroxysmal positional vertigo – toward new definitions. Otol Neurotol. 2014;35:323–328.
- Califano L, Vassallo A, Melillo MG, et al. Direction-fixed paroxysmal nystagmus lateral canal benign paroxysmal positioning vertigo (BPPV): another form of lateral canalolithiasis. Acta Otorhinolaryngol Ital. 2013;33:254–260.
- Buki B, Mandala M, Nuti D. Typical and atypical benign paroxysmal positional vertigo: literature review and new theoretical considerations. J Vestib Res. 2014;24:415–423.
- Buki B, Simon L, Garab S, et al. Sitting-up vertigo and trunk retropulsion in patients with benign positional vertigo but without positional nystagmus. J Neurol Neurosurg Psychiatry. 2011;82:98–104.
- Buttner U, Helmchen C, Brandt T. Diagnostic criteria for central versus peripheral positioning nystagmus and vertigo: a review. Acta Otolaryngol. 1999;119:1–5.