Abstract
The study aimed at describing a case of auditory brainstem implant (ABI) paediatric re-implantation performed at the Akademiska University Hospital, Sweden. The patient was a boy with Goldenhar syndrome with absent vestibular-cochlear nerves and was first implanted with an ABI in 2009 at the age of two years. A technical device failure in 2015 led to a re-implantation at the age of nine years. The ABI was successfully re-implanted although the implant was closely attached to the surrounding tissue and difficult to remove. The intraoperative electrical auditory brainstem measures (eABRs) gave unclear responses after re-implantation. After 12 months, the patient’s hearing thresholds was not as good as it was after the primary implant, but it is still developing. The child is a full-time user. ABI re-implantation is possible even after many years, although there is a risk that the implant might be fixed to the brainstem and difficult to remove.
Introduction
The first auditory brainstem implant (ABI) was inserted by House and Hitselberger in 1979. Edgerton et al. [Citation1] were the first to describe the ABI. At that time, only adult patients with neurofibromatosis type 2 (NF2) were selected to receive an implant. The implant allows patients to hear environmental sounds, monitor their own voice and experience enhanced lip reading [Citation2–4]. However, there have also been reports of NF2 patients who are able to hear open-set languages [Citation5]. There is evidence that the functional outcome from implanting non-tumour patients is much better compared to NF2 patients [Citation2]. This may be partly due to the brain plasticity in childhood [Citation6] and the cochlear nucleus (CN) not being distorted by a tumour as in NF2 patients [Citation7]. Since modern indications for ABIs also include non-tumour paediatric patients, the question of ABI re-implantation arises. Few ABI re-implantations have been performed globally, and a very limited number are described in literature. The largest series of ABI revision surgeries, mainly involving adults (n = 8), was published by Behr [Citation8]. In that study, most patients reported improvements in their hearing compared to their pre-revision situation.
This paper describes a single case of a paediatric ABI re-implantation performed at Akademiska University Hospital in Uppsala, Sweden. The surgery, fitting and functional outcome are described. This study was approved by the Uppsala Ethical Review Board (Dnr 2016/340) and informed written consent was given by the patient and parents.
Materials and methods
The patient was a boy with Goldenhar syndrome, who was first implanted on the right side with a Cochlear Nucleus 24 ABI from Cochlear Ltd. (Lane Cove, Australia) in 2009 at the age of two years. Prior to implantation, magnetic resonance imaging (MRI) showed absent vestibulocochlear nerve (eight cranial nerve, VIII) but as there was a small possibility of a thin partly functioning nerve, the patient was first operated with a cochlear implant (CI). The outcome was poor and an ABI was implanted. A technical device failure in 2015 led to an explanation of the Cochlear ABI and a re-implantation with a MedEl Synchrony ABI from MedEl GMBH (Innsbruck, Austria) at the age of nine years. The MedEl Synchrony ABI was used for the re-implantation due to the Cochlear ABI having been removed from the market for technical reasons. The functional outcome of the first implantation was earlier described by Siegbahn et al. [Citation9], Lundin et al. [Citation4], and the early functional outcome immediately after re-implantation was described by Lundin et al. [Citation10]. The principles for electrical auditory brainstem response measurements (eABRs) and processor fitting were described in Siegbahn et al. [Citation9] and Lundin et al. [Citation10].
Results
Primary implantation in 2009
Surgery
The surgery was performed using the translabyrinthine approach. The nervus vagus (tenth cranial nerve, X) was inspected and appeared to be normal. The nervus facialis (seventh cranial nerve, VII) and nervus intermedius were identified at the brainstem near the foramen Luschka. The vestibular-cochlear nerve could not be identified. The choroid plexus was used to identify the foramen Luschka. The electrode plate was inserted into the foramen Luschka with the glosso-pharyngeal nerve serving as a landmark for the entrance to the foramen Luschka. The position of the electrode plate had to be slightly modified based on the results from the electrophysiological measurements. The reference electrode was placed under the temporal muscle.
Electrophysiological measurements
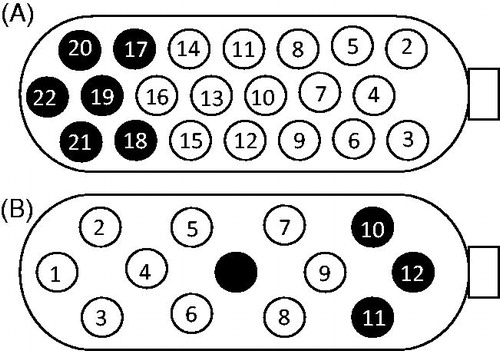
Intraoperative eABRs were recorded, and responses were obtained for all electrodes tested. In the majority of the recordings, a large negative potential was observed after the expected wave forms, which could be an indication of malstimulation with a major muscle response. Modification of the implant position did not affect the eABR responses. Repeated eABRs were recorded the day before fitting, eight weeks after implantation, in BP +5 mode. All tested combinations except two (electrode 15 and 16) gave positive responses. The electrode positions on the ABI plate are displayed in Figure . The large negative potential seen intraoperatively were also seen at this stage.
Figure 1. (A) Active electrodes on the implant from the first implantation. A black electrode indicates an electrode not in use. Size of the electrode plate: 3 × 8.5 mm. (B) Active electrodes on the implant from the re-implantation. A black electrode indicates an electrode not in use. Size of the electrode plate: 3 × 5.5 mm.
Fitting
No clear psychoacoustic responses were observed from the child at the initial fitting session. After two weeks, the child started to demonstrate responses to auditory stimuli. The reactions were unreliable for a long period of time, but the processor was worn while the child was awake. eABRs were re-evaluated after one year, and the levels were adjusted. The child had 15 channels activated in the map (15 is the maximum number of active channels in a BP +5 ABI map) and was programmed with the SPEAK strategy. Active electrodes on the implant are shown in Figure . The C-levels in the map varied between 210 and 245CL, and the pulse width was set to 100 μs.
Functional outcome
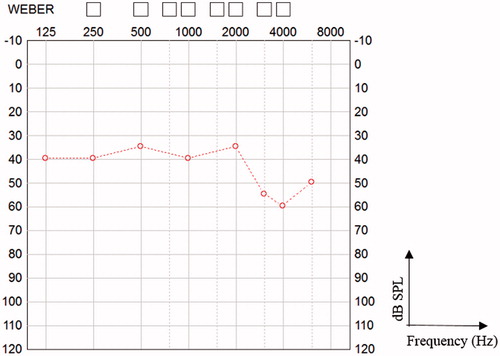
In 2015, six years after implantation, the child had difficulties reliably perceiving sounds above 3 kHz, but otherwise, he reacted consistently to ABI stimulation. Consonants were more difficult for him to perceive than vowels. Six years postoperatively, the category of auditory performance (CAP) [Citation11] score was 4. Hearing thresholds with the implant are shown in Figure . The child was a full-time user from the first day of implantation. In 2015, there was a technical device failure, and the implant ceased to function. The time to re-implantation was urgent for the child, since it was more difficult for him to communicate.
Re-implantation in 2015
Surgery
The translabyrinthine approach was used, and the failed device was first explanted. The cavity was partly obliterated by new bone growth and was drilled out. Fat tissue had organised into a connective tissue meshwork that was eliminated. Care was taken not to damage the gracile facial nerve in the open internal acoustic canal. The electrode cable of the failed implant had perforated the dura mater at three locations and had to be cut into pieces to be removed. The electrode cable was embedded in fibroid tissue, which was dissected against the foramen Luschka. The electrode mesh was fixed to the surrounding tissue. Meticulous dissection was performed and the electrode could finally be gently removed without damage to the surface tissue of the brain. Since a Cochlear® device was not available, a Med-El device was chosen. Before placement of the new implant, a Med-El ABI test device was used for measurements. The mesh of the new implant was reduced in size before implantation, and the electrode was fixed with soft tissue and fibrin glue. Both the test device and the ABI electrode positions were modified several times to determine the optimal location.
Electrophysiological measurements
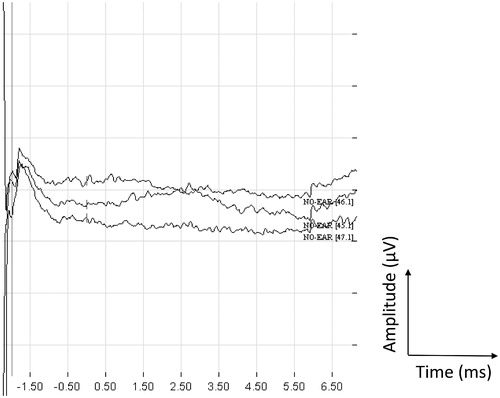
Intraoperative eABR measurements gave unclear responses. The Med-El ABI test device was first used, and the position was modified eight times. No responses were obtained from the measurements. After placement of the final ABI electrode and monitoring in bipolar mode, vague responses were recorded from electrodes 6 and 9. Monopolar stimulation provided ambiguous responses on electrodes 12 and 5. Eight weeks after implantation, on the day before the first fitting, eABRs were obtained from electrodes 2, 9 and 12. Recordings from stimulating electrode 2 at 48 qu in positive, negative and alternating modes are shown in Figure . Electrodes 5, 7, 10 and 11 gave vague responses, but no responses were received from electrodes 1, 3, 4, 6 and 9. The electrode positions on the ABI plate are displayed in Figure .
Fitting
On the day of the first fitting, the child responded to stimulation on eight electrodes. Three months after fitting, the child presented reliable responses to stimulation on all electrodes except 11 and 12. Those electrodes were turned off. Later, electrode 10 was also turned off. At 12 months, the patient detected stimulation at 60% of C-level on all nine remaining active electrodes. The C-levels vary between 25 and 58 qu. The minimal pulse width was set to 40 μs. Active electrodes on the implant are shown in Figure .
Functional outcome
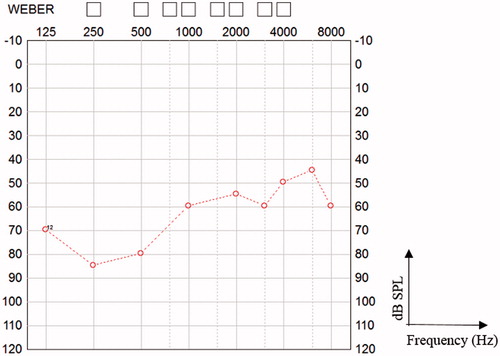
The child has been a full-time user from the first day of implantation. The CAP score reached 4 after 12 months, but the boy’s family believe that hearing was better with the first device. He has difficulties to reliably perceive low frequency sounds. Auditory thresholds 12 months following re-implantation are shown in Figure . Considering the difficulties encountered at revision surgery, intraoperative eABRs and general condition, the results are promising, and further improvement is expected.
Discussion
Currently, revision ABI surgery has been carried out in only a limited number of patients [Citation12]. In some cases, the electrode could be easily tracked, dissected and removed, but in other cases, the electrode was fixed to the brainstem and could not be dissected out. The leading causes for revision surgery are device failure, electrode migration or tumour regrowth in NF2 cases. Puram et al. [Citation13] described one case of ipsilateral re-implantation after six months. Scar tissue was dissected around the dura, the implant was removed and a new device was inserted. The mesh backing the electrode array was removed during the primary surgery. They concluded that re-implantation can be successful without any significant adverse outcomes. The number of active electrodes remained the same after re-implantation (15 and 14, respectively). The non-auditory side effects were less, and the pure tone average (PTA) was better after the re-implantation when compared with the primary surgery. In their study of four paediatric patients, they experienced three device failures, of which two occurred in the same patient. However, only one re-implantation was described in their study. Manrique et al. [Citation14] implanted 12 cynomolgus macaques with dummy ABIs and showed that the ABI caused minimal damage to the CN if the surgical procedure was carefully undertaken. The mesh was not considered to be a contraindication for re-implantation, although it had to be detached or left in place when removing the implants in four of the six cases. Lenarz et al. [Citation15] described one case of revision surgery, due to electrode migration, three months after the primary surgery. Only minor scar formation was found, and the implant could be removed and successfully repositioned. In our case, the ABI was closely attached to the surrounding tissue and was difficult to remove. The mesh was fixed to the surrounding tissue to prevent electrode dislocation. The number of active electrodes after re-implantation could not easily be compared since two different ABI types were used. Auditory thresholds were worse with the second implant.
The duration between the primary and second surgery may play a role in the fixation of the electrode array. A re-implantation within 3–6 months may not have led to electrode fixation and scar tissue formation around the electrode. However, cases have been described in which there was less scar tissue even after several years [Citation8]. The indication to revise is a clear-cut change in eABRs, psychoacoustic threshold increase and CT examination suggesting electrode migration. Migration of the electrode plate occurs most frequently within the first three months, presumably when the scar tissue is still under formation.
Ramsden et al. [Citation16] described two revision cases with repositioning of the electrode plate. In both cases, there was dense scar tissue around the array and a combination of blunt and sharp dissection was required to mobilise it. Furthermore, they speculated that revision surgery may be easier with a smaller array and mesh. Behr [Citation8] also reported that the larger devices may be more difficult to remove at revision. He concluded that revision in case of tumour growth, or a complete exchange of ABI systems in technical failure cases, can be performed without neurological sequelae.
If the electrode plate is fixed to the brainstem, the vulnerable structures of the CN may be easily damaged. Fibrosis development between the electrode plate and the brainstem may also prevent stimulation. It seems unlikely that the secondary implant will stimulate the same topically arranged neurons of the CN. In the present case, hearing for low frequency sounds was better after the primary implantation possibly suggesting that a slight over-insertion, whilst after re-implantation high-frequency hearing was better suggesting a slight under-insertion. This could explain the parent’s view that hearing with the second implant was not yet in parity with the first device, even though he reaches the same CAP-score. The change of ABI manufacturer and stimulation strategy could also influence sound perception and adaptation. Furthermore, the size of the electrode plate on the implants differ, the first implant had the dimension 3 × 8.5 mm and the second implant was shorter, 3 × 5.5 mm. New electrode designs and re-implantation without the need for electrode plate replacement would be beneficial and less demanding to the CN. Such a solution may meet other technical challenges though [Citation12].
Conclusions
We present one case of paediatric re-implantation of an ABI device due to technical failure after six years. Results suggest that re-implantation is possible even many years after the primary surgery. There seems to be an increased risk for electrode fixation to the brainstem, which makes it difficult to remove without damage to the brain stem and feeding vessels to the CN. After 12 months the second device operates well and hearing seems to be improving.
Acknowledgements
The patient’s primary implantation was performed under the supervision of Professor Vittorio Colletti, Verona, Italy, and the reoperation was performed by Professor Robert Behr, Fulda, Germany.
Disclosure statement
Professor Helge Rask-Andersen receives partial research funding from the Med-El company in Innsbruck, Austria. The Cochlear Nucleus 24 ABI device is not labelled for use in individuals below the age of 12, and the MedEl Synchrony ABI is not labelled for use in individuals below the age of 15.
Additional information
Funding
References
- Edgerton BJ, House FW, Hitselberger WE. Hearing by cochlear nucleus stimulation in humans. Ann Otol Rhinol Laryngol Suppl. 1982;91:117–124.
- Colletti L, Zoccante L. Nonverbal cognitive abilities and auditory performance in children fitted with auditory brainstem implants: preliminary report. Laryngoscope. 2008;118:1443–1448.
- Grayelin AB, Kalamarides M, Bouccara D, et al. Auditory brainstem implant in neurofibromatosis type 2 and non-neurofibromatosis type 2 patients. Otol Neurotol. 2008;29:1140–1146.
- Lundin K, Stillesjö F, Nyberg G, et al. Self-reported benefit, sound perception, and quality-of-life in patients with auditory brainstem implants (ABIs). Acta Otolaryngol. 2015;136:62–67.
- Behr R, Colletti V, Matthies C, et al. New outcomes with auditory brainstem implants in NF2 patients. Otol Neurotol. 2014;35:1844–1854.
- Shannon RV. Auditory implant research at the House Ear Institute 1989–2013. Hear Res. 2015;322:57–66.
- Sanna M, Khrais T, Guida M, et al. Auditory brainstem implant in a child with severely ossified cochlea. Laryngoscope. 2006;116:1700–1709.
- Behr R. Revision surgery in auditory brain stem implantation. J Neurol Surg B. 2015;76:A111.
- Siegbahn M, Lundin K, Olsson G-B, et al. Auditory Brainstem Implants (ABIs)-20 years of clinical experience in Uppsala, Sweden. Acta Otolaryngol. 2014;134:1052–1061.
- Lundin K, Stillesjö F, Nyberg G, et al. Experiences from auditory brainstem implantation (ABI) in four paediatric patients. Cochlear Implants Int. 2016;17:109–115.
- Archbold S, Lutman ME, Nikolopoulos T. Categories of auditory performance: inter-user reliability. Br J Audiol. 1998;32:7–12.
- Sennaroglu L, Colletti V, Lenarz T, et al. Consensus statement: long-term results of ABI in children with complex inner ear malformations and decision making between CI and ABI. Cochlear Implants Int. 2016;17:163–171.
- Puram SD, Barber SR, Kozin ED, et al. Outcomes following pediatric auditory brainstem implant surgery: early experiences in a North American Center. Otolaryngol Head Neck Surg. 2016;155:133–138.
- Manrique M, Jauregui I, Insausti A, et al. Experimental study following inactive implantation of an auditory brain stem implant in nonhuman primates. Ann Otol Rhinol Laryngol. 2000;109:163–169.
- Lenarz T, Moshrefi M, Matthies C, et al. Auditory brainstem implant: part I. Auditory performance and its evolution over time. Otol Neurotol. 2001;22:823–833.
- Ramsden RT, Freeman SRM, Lloyd SKW, et al. Auditory brainstem implantation in neurofibromatosis type 2: experience from the manchester programme. Otol Neurotol. 2016;37:1267–1274.