Abstract
Takayasu arteritis (TAK) is a rare type of noninfectious, systemic vasculitis of unknown etiology. A 45-year-old female diagnosed with TAK complained of acute onset bilateral deafness. Auditory tests revealed complete deafness in both ears. Magnetic resonance imaging with fluid-attenuation inversion recovery of her temporal bone demonstrated high signal intensity in the whole cochlea and vestibule in both ears. Computed tomography carried out 10 months after the onset of bilateral deafness revealed prominent ossification localized in the area around the modiolus in both cochleae. The patient underwent cochlear implantation in both ears. The cochlear lumens were narrowed and filled with scar tissue, which hindered the insertion of an electrode in both ears. In TAK, cases associated with bilateral severe to profound SNHL where corticosteroid therapy is ineffective in improving hearing, cochlear implantation should be considered within a reasonable period to avoid the difficulty of electrode insertion associated with cochlear ossification.
Introduction
Takayasu arteritis (TAK), which was first described by Takayasu in 1908, is a noninfectious, systemic vasculitis of unknown etiology. The disease is commonly seen in women in the second or third decade of life [Citation1]. TAK predominantly affects the aorta and/or its branches, and most of the major manifestations of TAK come from limb or organ ischemia resulting from the stenosis of the involved arteries [Citation2]. Sensorineural hearing loss (SNHL) is a well-recognized manifestation of various systemic vasculitides, such as antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides [Citation3,Citation4] and Cogan’s syndrome [Citation2]; however, in patients with TAK, only a few cases of SNHL have been reported in the English literature. Accordingly, the pathogenesis remains poorly understood, and there is no established treatment strategy for SNHL [Citation5–11]. We herein report the clinical findings in a patient with TAK who developed sudden bilateral deafness and underwent bilateral cochlear implants. The magnetic resonance imaging (MRI) findings during the acute phase of hearing loss and the intraoperative findings of the patient may shed light on the pathogenesis of SNHL associated with TAK.
Case report
A 45-year-old female with acute onset bilateral deafness and vertigo was referred to our university hospital. One year before the onset of hearing loss, the patient had visited a general practitioner with complaints of abdominal pain and backache. She was diagnosed with TAK based on the findings of thoracoabdominal contrast-enhanced computed tomography (CT), which revealed a thickened wall of the descending aorta, along with elevated C-reactive protein levels. After five-month remission with prednisolone and methotrexate, the patient experienced recurrent left hearing loss, tinnitus, and vertigo and was diagnosed with Meniere’s disease. After nine-month remission, she had epigastric pain and again underwent thoracoabdominal contrast-enhanced CT, which revealed relapse of TAK. After the administration of tocilizumab (a humanized monoclonal antibody against the interleukin-6 receptor) in addition to prednisolone and methotrexate, the patient seemed to obtain remission at least for three months. However, almost one year after the onset of TAK, she experienced common cold-like symptoms followed by bilateral hearing loss and vertigo without other neurological defects. Over the next couple of days, she completely lost her hearing in both ears.
Five days after the onset of hearing loss, the patient was referred to our university hospital. An otoscopic examination revealed normal tympanic membranes. A pure-tone audiogram showed bilateral profound SNHL without any residual hearing. The speech discrimination scores were 0% bilaterally. Click-evoked auditory brainstem responses exhibited no response in either ear at a maximum stimulus intensity of a 105 dB normal hearing level. The distortion product otoacoustic emission amplitudes were decreased to the noise levels bilaterally. Spontaneous leftward horizontal nystagmus was observed under a charge-coupled device (CCD) camera with infrared illumination, and caloric testing showed bilateral almost complete canal paresis. Laboratory tests revealed a slightly elevated white blood cell count of 10,000/mm3, a normal range of C-reactive protein and negative findings for proteinase 3- and myeloperoxidase-antineutrophilic cytoplasmic antibodies.
MRI of the temporal bone was performed five days after the onset of hearing loss. T2-weighted imaging of the inner ears showed high signal intensity equivalent to the signal intensity of the cerebrospinal space, and post-contrast T1-weighted imaging showed no abnormal enhancement (Figure ). It was striking that noncontrast fluid-attenuation inversion recovery (FLAIR) revealed markedly high signal intensity in the whole cochlea and vestibule in the left ear and slightly high intensity in the right ear (Figure ). MR angiography showed a normal appearance of the vertebrobasilar artery: the anterior inferior cerebellar artery (AICA) was visualized on the left side (Figure ). CT performed six days after the onset of hearing loss showed no apparent abnormalities within the fluid spaces of the membranous labyrinth (Figure ). One month after the onset of hearing loss, MRI T2-weighted images and fast imaging employing steady-state acquisition (FIESTA) images still showed high signal intensity in the inner ears, while the signal intensity on non-contrast FLAIR was decayed compared to that in the early phase of hearing loss (Figure ).
Figure 1. MRI of the temporal bones. Five days after the onset of hearing loss, axial T2-weighted images show high signal intensity from the fluid in both ears. Noncontrast fluid-attenuated inversion recovery (FLAIR) images show markedly high signal intensity in the whole cochlea and vestibule in the left ear and slightly high signal intensity in the right ear. Gadolinium-enhanced T1-weighted (Gd-T1) images show no abnormal enhancement. One month after the onset of hearing loss, the signal intensity on noncontrast FLAIR was decayed compared to that in the early phase of hearing loss. High signal intensity on T2-weighted image and three-dimensional fast imaging employing steady-state acquisition (FIESTA) images indicate the presence of fluid in both cochleae.
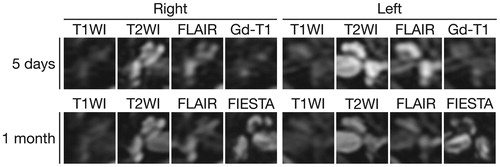
Figure 2. MR angiography. MR angiography shows the normal appearance of the vertebrobasilar artery. The anterior inferior cerebral artery (AICA; arrowheads) is visualized on the left side. The lack of visualization of the AICA does not necessarily imply occlusion of the vessel.
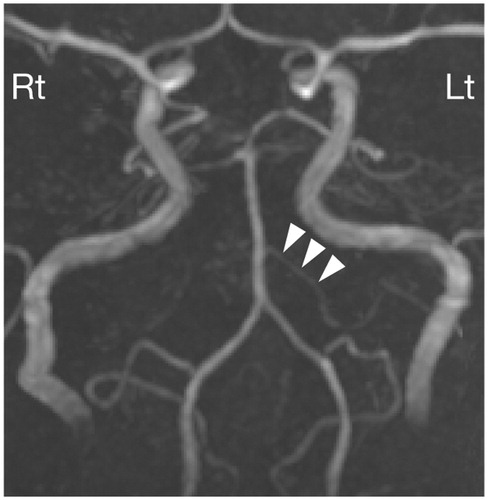
Figure 3. CT of the temporal bones. Axial CT images six days after the onset of hearing loss show the normal appearance of the inner ear bilaterally. Axial CT images of the temporal bone on the first postoperative day which was 10 months after the onset of hearing loss reveal prominent cochlear ossification around the modiolus in both cochleae. LSCC: lateral semicircular canal.
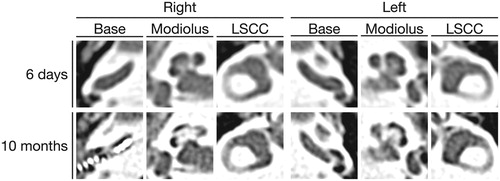
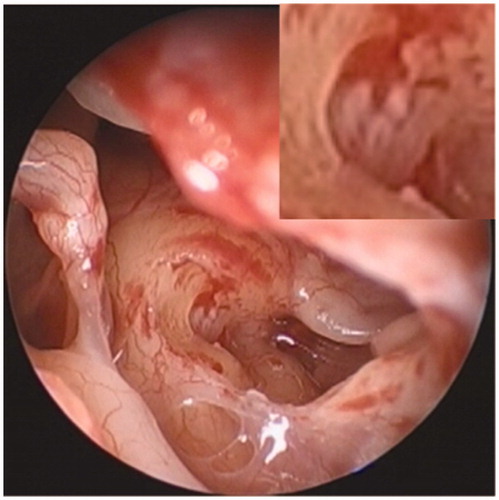
Steroid therapy was initiated with the intravenous administration of 10 mg of betamethasone and the oral administration of 50 mg prednisolone, the doses of which were then tapered after 10 days. She also received hyperbaric oxygen therapy and intratympanic injections of dexamethasone as salvage therapy; however, her hearing did not recover in either ear. The patient underwent cochlear implantation in the right ear 10 months after the onset of bilateral deafness. The round window membrane was white-colored and thickened (Figure ), and the narrowed cochlear lumen filled with scar tissue hindered electrode insertion through the round window. Drilling was therefore performed above the basal lesion of the scala tympani, and partial electrode insertion was performed. CT carried out on the first postoperative day showed prominent ossification around the modiolus in both cochleae (Figure ). Since the patient did not achieve open-set speech perception post-implantation, cochlear implantation in the left ear was also planned 15 months after the onset of bilateral deafness. The intraoperative finding in the left cochlea was similar to that in the right cochlea, and an electrode was again partially inserted. The patient still had poor speech perception even three months after bilateral cochlear implantation.
Discussion
To our knowledge, this is the first report describing FLAIR-MRI of the inner ear in the early phase of sudden bilateral deafness associated with TAK as well as the intraoperative findings of cochlear implantation. TAK was previously thought to be more prevalent in Asian countries than in other regions; however, it has been reported at similar incidence rates of 1–2 per million in most parts of the world [Citation12]. Due to the scarcity of TAK cases, whether the hearing loss that occurs in patients with TAK is really a rare manifestation or in fact a common manifestation that has only rarely been reported is unclear. Recently, Yasui and Yamasoba [Citation9] described two of their own patients and reviewed 27 previously reported cases of SNHL associated with TAK. Of these 29 cases, 25 (86%) were female, and 23 (79%) were in the fourth and fifth decades of life. Of the 20 cases where the affected side was described, 18 (90%) showed bilateral hearing loss, while only two cases showed unilateral hearing loss. In most of the cases, the audiogram showed a sloping or falling configuration, and two cases were deaf.
The pathogenesis of SNHL associated with TAK remains a matter of speculation. Since most of the major manifestations of TAK come from limb or organ ischemia resulting from the stenosis of the involved arteries [Citation2], and since TAK can affect medium-sized and even small arteries [Citation2], ischemia of the inner ear stemming from an occlusive lesion of the feeding arteries is certainly possible [Citation5]. Nomura and Kitamura [Citation13] reported the temporal bone histopathology of a case of bilateral SNHL with a falling configuration who was suffering from TAK. The main histopathological findings were the lack of inner and outer hair cells in the basal turn and the marked loss of cochlear neurons in the same region. Remarkably, the blood vessels within the cochlea and the internal auditory meatus were normal. In our case, MR angiography showed a normal appearance of the vertebrobasilar artery, including the clearly visualized AICA, at least on the left side. These findings seem to disclaim ischemia of the inner ear as the pathogenesis; however, we cannot exclude the possibility that microvascular ischemia, the findings of which would not be seen on MRI, might have caused hearing loss in the present case.
Another possible cause is the immune-mediated inflammatory process in the membranous labyrinth [Citation5,Citation9]. Kanzaki et al. [Citation10] reported six cases of steroid-dependent SNHL associated with TAK. Yasui and Yamasoba [Citation9] also reported that corticosteroid therapy was effective in improving the hearing in 14 out of 20 cases of SNHL associated with TAK. In our case, although corticosteroid therapy was ineffective after the onset of severe bilateral hearing loss, antecedent Meniere’s disease-like symptoms seemed to be partially controlled with prednisolone and methotrexate. Circulating immune complexes have been reported in cases of SNHL associated with TAK [Citation5,Citation14]. There is evidence suggesting that strial capillaries may be a target of autoimmune hearing disorders [Citation15]. For example, human temporal bone studies of hearing loss in Sjogren’s syndrome revealed immunoglobulin G deposition in the basement membrane of the stria vascularis [Citation16]. Disrupted vascular integrity in the stria vascularis changes the blood barrier permeability in the stria vascularis [Citation15]. In our case, noncontrast FLAIR revealed high signal intensity in the whole cochlea and vestibule (Figure ), which might reflect an increased concentration of protein passing through blood vessels with increased permeability [Citation17]. These findings support the possibility that SNHL in patients with TAK is caused by the autoimmune response in the membranous labyrinth.
There are limited number of studies examining cochlear implantation in patients deafened by suspected autoimmune inner ear disease. Wang et al. [Citation18] reported 18 patients with autoimmune inner ear disease. Intraoperatively, one patient was found to have ossification of the basal turn of the scala tympani, and partial electrode insertion was performed. To our knowledge, there is only one report in the Japanese literature on cochlear implantation in a patient with TAK. Aoki et al. [Citation19] encountered a 65-year-old female with bilateral deafness associated with TAK. The hearing loss failed to improve with corticosteroid therapy, and the patient underwent cochlear implantation one year after the onset of deafness. Intraoperatively, the patient was found to have cochlear ossification a few millimeters from the round window membrane, yet full electrode insertion into the scala tympani was achieved, and the patient recovered 90% of open-set syllable recognition scores.
In conclusion, the clinical findings of our case and previously reported cases support an autoimmune response in the membranous labyrinth as the pathogenesis of hearing loss in patients with TAK, even though currently available information cannot exclude the possibility that microvascular ischemia can cause hearing loss. In cases where corticosteroid therapy is ineffective in improving hearing, cochlear implantation should be considered within a reasonable period to avoid the difficulty of electrode insertion associated with cochlear calcification.
Patient consent
We followed the ICMJE requirements on privacy, and written consent for publication was obtained from a patient.
Acknowledgements
The authors of the above article have no acknowledgements to include.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Johnston SL, Lock RJ, Gompels MM. Takayasu arteritis: a review. J Clin Pathol. 2002;55:481–486.
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11.
- Takagi D, Nakamaru Y, Maguchi S, et al. Otologic manifestations of Wegener's granulomatosis. Laryngoscope. 2002;112:1684–1690.
- Nakamaru Y, Takagi D, Oridate N, et al. Otolaryngologic manifestations of antineutrophil cytoplasmic antibody-associated vasculitis. Otolaryngol Head Neck Surg. 2012;146:119–121.
- Siglock TJ, Brookler KH. Sensorineural hearing loss associated with Takayasu's disease. Laryngoscope. 1987;97:797–800.
- Raza K, Karokis D, Kitas GD. Cogan's syndrome with Takayasu's arteritis. Br J Rheumatol. 1998;37:369–372.
- Maruyoshi H, Toyama K, Kojima S, et al. Sensorineural hearing loss combined with Takayasu’s arteritis. Intern Med. 2005;44:124–128.
- Smith JC, Peck JE, Ray LI, et al. Aortoarteritis and sensorineural hearing loss in an adolescent black male. Am J Otolaryngol. 2004;25:370–376.
- Yasui T, Yamasoba T. Acute sensorineural hearing loss associated with aortitis syndrome. Acta Otolaryngol Suppl. 2007;559:29–33.
- Kanzaki J, Kanzaki S, Ogawa K. Long-term prognosis of steroid-dependent sensorineural hearing loss. Audiol Neurootol. 2009;14:26–34.
- Kunihiro T, Kanzaki J, O-Uchi T, et al. Steroid-responsive sensorineural hearing loss associated with aortitis syndrome. ORL J Otorhinolaryngol Relat Spec. 1990;52:86–95.
- Richards BL, March L, Gabriel SE. Epidemiology of large-vessel vasculidities. Best Pract Res Clin Rheumatol. 2010;24:871–883.
- Nomura Y, Kitamura K. Abrupt (sharp cut) type sensorineural hearing loss-a human temporal bone study. Auris Nasus Larynx. 1979;6:13–21.
- Kanzaki J, O-Uchi T. Circulating immune complexes in steroid-responsive sensorineural hearing loss and the long-term observation. Acta Otolaryngol Suppl. 1983;393:77–84.
- Shi X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear Res. 2016;338:52–63.
- Calzada AP, Balaker AE, Ishiyama G, et al. Temporal bone histopathology and immunoglobulin deposition in Sjogren's syndrome. Otol Neurotol. 2012;33:258–266.
- Sugiura M, Naganawa S, Teranishi M, et al. Three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging findings in patients with sudden sensorineural hearing loss. Laryngoscope. 2006;116:1451–1454.
- Wang JR, Yuen HW, Shipp DB, et al. Cochlear implantation in patients with autoimmune inner ear disease including cogan syndrome: a comparison with age- and sex-matched controls. Laryngoscope. 2010;120:2478–2483.
- Aoki M, Yokota Y, Ando K, et al. Cochlear implantation in a patient with Takayasu's arteritis. Practica Otologica. 2006;99:275–278.