Abstract
Introduction: Solitary fibrous tumor/hemangiopericytoma is an uncommon neoplasm that accounts for only 1–2% of all soft tissue tumors. To the best of our knowledge only three cases of this tumor have been described in the external auditory canal in the past.
Case presentation: We report the case of a 60 years old man with a 6 months history of progressive swelling of the left auditory canal. After resection under general anaesthesia, the immunohistochemical staining showed positivity to CD34, CD99, vimentin and bcl2 with the typical spindle-shaped cells organized in a patternless pattern. The tumor was also positive to specific STAT6 nuclear expression. Therefore, the diagnosis of solitary fibrous tumor/hemangiopericytoma was made.
Conclusions: The possibility of a STF/HPC should be considered among the differential diagnosis of tumors in the external auditory canal and, due to its malignant potential, a close and long follow up should be done in all the patients.
Introduction
Solitary fibrous tumor (SFT) is a rare mesenchymal neoplasm first described in 1931 by Klemperer and Rabin in the pleura [Citation1]. In the past, it has been classified as a different but related entity to hemangiopericytomas, both having intermediate malignant potential, as described by the World Health Organization (WHO) classification scheme [Citation2]. Currently, they have been categorized as different manifestations of the same pathology under the combined term ‘solitary fibrous tumor/hemangiopericytoma’ (SFT/HPC) by the WHO classification of tumors of the central nervous system in 2016 [Citation3]. We report a case of a solitary fibrous tumor in the external auditory canal (EAC) in a 60-year-old male. To the best of our knowledge, there have been only three cases reported in the past by Izumaru et al. [Citation4], Rezk et al. [Citation5], and Lee et al. [Citation6]. We describe the fourth case of SFT in the EAC and a review of the literature.
Case report
A 60-year-old male, smoker, with no previous health conditions, presented to our hospital with a 7-month history of progressive swelling, intermittent suppuration and hearing loss of the left ear. Physical examination revealed a soft and collapsible bulging on the posterior wall of the external third of the left EAC, occluding it almost completely. Once collapsed, the middle ear looked normal and the audiometry showed a mild mix hearing loss with a predominant conductive component due to the obstruction caused by the tumor.
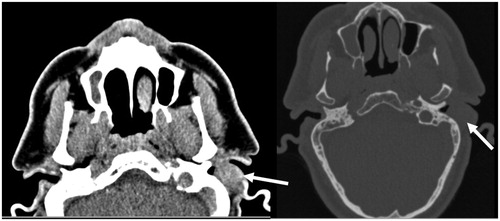
A Computed Tomography Scan (CT-scan) revealed a round, homogeneous soft-tissue mass on the posterior wall of the EAC with extension and remodeling of its bony portion. No necrosis or bone erosion was seen (Figure ).
Figure 1. Computed tomography shows a homogenous and well circumscribed soft tissue mass in the left external auditory canal (white arrows).
The tumor was excised under general anesthesia. Macroscopically, it was a round, elastic, well circumscribed, not encapsulated nodule with a gray-whitish color, measuring 2 × 2 × 1 cms (Figure ). Microscopically, the tumor showed hypo- and hyper-cellular areas of spindle cells, separated by thick queloid-like hyalinized collagen bands distributed in a ‘patternless pattern’. Some areas of myxoid degeneration were also seen. No necrosis, significant atypia, nor invasion of the surrounding tissue was observed and there were less than 3 mitosis per 10 high-power fields (HPF) (Figure ).
Figure 2. Surgical exploration showed a well circumscribed, not encapsulated soft tissue mass of 2 × 2 × 1 cms.
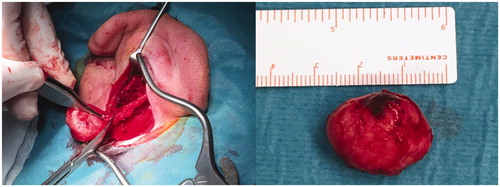
Figure 3. Histological sections. (3a): Patternless pattern of hyper and hypocelullar areas separated by collagen bands (Hematoxylin and Eosin (H&E) magnification 10×); (3b): hypercelullar area with spindle-like cells (H&E magnification 20×). On immunohistochemical analysis; (3c): tumor cells were highly reactive to CD34 (magnification 10×); (3d): CD99 (magnification 20×); (3e): Bcl-2 (magnification 10×); (3f): vimentin (magnification 10×); (3g): Positive STAT6 nuclear expression (magnification 10×).
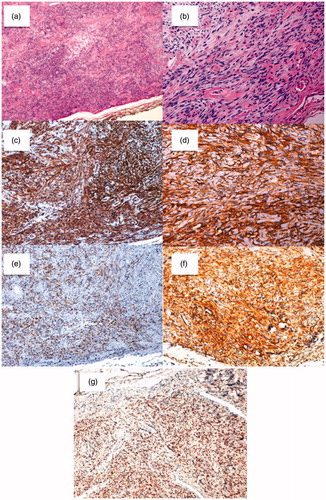
Inmunohistochemically, tumor cells showed positivity to CD34, CD99, Vimentin, and bcl2. They had a negative reaction to s100, desmin, actin HHF35, and actin 1A4. The tumor was also positive to STAT6 nuclear expression (Figure ).
Based on these findings and specific STAT6 nuclear expression, the diagnosis of SFT/HPC was made. After two years of follow-up, the patient has not shown any signs of recurrence.
Discussion
SFT/HPC is a rare neoplasm that commonly develops in middle age adults from 20 to 80 years old, with a median age between 40 and 50 years and equal male/female distribution [Citation2]. This tumor has three classic primary locations: pleural, meningeal, and extrathoracic soft tissue and it accounts for a 1–2% of all soft tissues tumors [Citation1]. In the head and neck it has been commonly described in the orbit, nasal cavity, oral cavity, larynx, thyroid, salivary glands, parapharyngeal space, among others, with an incidence of approximately12–20% [Citation7,Citation8]. In the external auditory canal, there have been only three reported cases in the past [Citation4–6].
The radiographic characteristics of SFT/HPC on CT and magnetic resonance imaging (MRI) are similar to those of other soft tissue tumors [Citation8]. On CT-scan, SFT/HPC appears as a well-defined, normally homogeneous isodense mass, and shows moderate to marked enhancement following contrast administration [Citation8]. MRI shows a low-signal intensity in T1 and a variable T2 signal, which correlates with their fibrous nature. Highly collagenized and hypocellular tumors have a low intensity in T2 while tumors with necrosis, myxoid degeneration, perivascular edema, and hypercellular content show a high-signal intensity [Citation4,Citation8].
In the past, the SFT and the hemangiopericytoma were considered two different but related entities with a blurred border between them, until the discovery of a shared inversion at 12q13 that leads to STAT6 nuclear expression due to the fusion between NAB2 and STAT6 genes [Citation9,Citation10]. Several genetic variants of this fusion have been described. Around 70–90% of the cases have the NAB2ex4-STAT6ex2/3 fusion variant, which normally characterizes pleural SFTs and a benign behavior. NAB2ex6-STAT6ex16/17 is the second variant in frequency and is usually found in cases with extrathoracic location, more aggressive behavior and malignant histology [Citation11]. Consequently, the 2016 WHO Classification of Tumors of the Central Nervous System united both entities under the name ‘solitary fibrous tumor/hemangiopericytoma’ giving it a classical three grades classification, based primarily on the histological findings. Grade I corresponds to the typical findings of the previously described ‘solitary fibrous tumor’, with its ‘patternless pattern’ of a low-cellularity spindle cells lesion separated by highly collagenous queloid-like bands. Grade II corresponds to the previous diagnosis of ‘hemangiopericytoma’, with a less collagenous, higher cellularity tumor with plump cells and ‘staghorn’ vasculature. Grade III compels to any of the previous descriptions with 5 or more mitoses per 10 high-power fields. [Citation2,Citation3,Citation8].
The cells in a SFT/HPC are normally immunoreactive for CD34 (90–95%) and CD99 (70%), as well as a variably positive reaction to Bcl2 and vimentin [Citation1,Citation2,Citation8]. These tumor cells also show a negative reaction to s-100, smooth muscle actin and desmin, as in the present case [Citation10,Citation11].
Although SFT/HPC usually pursue a benign course, approximately a 10–30% of them behave aggressively [Citation1,Citation2,Citation8]. The prognostic factors normally related to a malignant behavior are large size, high cellularity, increased mitotic activity, necrosis or hemorrhage, pleomorphism, stromal or vascular invasion, and the NAB2ex6-STAT6ex16/17 fusion variant found in extrathoracic SFTs [Citation1,Citation2,Citation8,Citation11]. The tumors classified as malignant tend to metastasize in 10–40% of the cases in five years and both benign and malignant lesions can reappear after 20 years [Citation1,Citation8]. Our patient did not show any of these signs and thus, the diagnosis of benign (grade I) STF/HPC was given.
En-bloc surgery for treatment of both benign and malignant STF/HPC is recommended. If the tumor shows signs of malignancy or a positive margin is found after resection, treatment with adjuvant radiotherapy should be considered. The need for preoperative embolization should also be assessed before surgery [Citation1,Citation8]. None of the cases reported previously in the EAC showed an aggressive behavior and only one of them needed preoperative embolization [Citation6]. In spite of its normally benign course, a particularly careful followup should be pursued in all patients with extrathoracic SFT due to the genetic variations that give them a potential higher risk of aggressive behavior.
Conclusions
The possibility of a STF/HPC should be considered among the differential diagnosis of tumors in the external auditory canal. Despite the recent advances in the genetic and molecular characterization of this tumor, it is not yet possible to predict its behavior accurately. The different variant in the NAB2-STAT6 fusion pattern found in extrathoracic SFT seems to give these cases a higher potential of malignancy [Citation11] and thus a close and long followup should be done in all the patients.
Disclosure statement
All authors have equal contribution and no conflicts of interest to disclose.
References
- Penel N, Amela E, Decanter G, et al. Solitary fibrous tumors and so-called hemangiopericytoma. Sarcoma. 2012;2012:1–6.
- Fletcher C DM, Unni KK, Mertens F. Pathology and genetics of tumours of soft tissue and bone. WHO classification of tumours. IARC. 2002;5:1–6.
- Louis D, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820.
- Izumaru S, Yoshida Y, Nakashima T. A solitary fibrous tumor in the external auditory meatus. Auris Nasus Larynx. 2004;31:65–67.
- Rezk S, Yousef M, Zamansky M, et al. Solitary fibrous tumor of the auditory canal. Arch Pathol Lab Med. 2004;128:169–171.
- Lee C, Lee H. Is a solitary fibrous tumor in the external auditory canal benign? J Audiol Otol. 2016;20:120–122.
- Demicco E, Park M, Araujo D, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25:1298–1306.
- Demicco EG. Solitary fibrous tumor. In: Basow, DS, editor. UpToDate. Waltham, MA: Meyer C.; 2017.
- Chmielecki J, Crago A, Rosenberg M, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013;45:131–132.
- Robinson D, Wu Y, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45:180–185.
- Ronchi A, Cozzolino I, Zito Marino F, et al. Extrapleural solitary fibrous tumor: a distinct entity from pleural solitary fibrous tumor. An update on clinical, molecular and diagnostic features. Ann Diagn Pathol. 2018;34:142–150.
