Abstract
In patients with cervical necrotizing fasciitis undergoing extensive surgical debridement, vascularized reconstruction may be required. Little is known regarding the outcomes for free tissue transfer in the setting of a prior necrotizing infection in the head and neck. A retrospective chart review identified patients treated for cervicofacial necrotizing fasciitis at a single academic tertiary care center between 2008 and 2017. Disease and functional data were collected on patients requiring free tissue transfer. Among 23 patients treated for necrotizing fasciitis of the head and neck, two patients required free tissue transfer (each with an anterolateral thigh free flap) after appropriate control of their infectious process. There were no postoperative complications and both patients recovered well with good functional outcomes. These cases demonstrate that in select patients with extensive cervicofacial soft tissue defects after resolution of necrotizing fasciitis, free tissue transfer may be a safe and viable option.
Introduction
Necrotizing fasciitis is a rapidly spreading infection, involving fascia and soft tissue, leading to necrosis of affected tissues and overlying skin. If not promptly recognized and treated, this disease can be fatal in 18–50% of patients [Citation1,Citation2] with higher mortality when the mediastinum is involved. It may affect any area of the body, but it is most common in the extremities, trunk or abdomen following trauma or surgery [Citation3,Citation4]. Necrotizing fasciitis in the head and neck is rare but is well described, most commonly following odontogenic, tonsillar, or pharyngeal infections, surgery, or self-administered injection [Citation5–7]. It is also more frequently seen in patients with co-morbidities such as diabetes mellitus, obesity, hypertension, tobacco use, or immunocompromise [Citation8,Citation9]. The main treatment modality is aggressive surgical debridement with supportive care and antimicrobial therapy [Citation9,Citation10]. Such debridement, however, may lead to significant tissue loss, requiring vascularized reconstruction for optimal post-treatment function. However, specific challenges arise in reconstruction after necrotizing fasciitis, due to surrounding inflammation, tissue edema, and vessel depletion, often severely limiting otherwise optimal reconstructive options. There is a paucity of reports on free tissue reconstruction in the setting of extensive cervical necrotizing fasciitis and the surgical outcomes and prognostic factors remain uncertain [Citation10].
Methods
A retrospective chart review was performed identifying patients treated for cervicofacial necrotizing fasciitis between 2008 and 2017 at Washington University in St. Louis. Among 23 patients, two had resultant tissue defects that required free tissue transfer. Data was collected on patient demographics, disease management and functional outcomes. All data collection and analysis was approved by the Washington University School of Medicine Institutional Review Board approval.
Cases
Case 1
A 28-year-old male with a past medical history of Tetrology of Fallot presented with a rapidly progressive deep neck space infection. He had no history of immunosuppression, diabetes, IV drug use, or other risk factors for severe necrotizing infection, and was not on any chronic medications prior to arrival. Imaging was notable for extensive soft tissue gas involving multiple deep neck space compartments and extending to the chest wall, consistent with necrotizing fasciitis. Multiple peri-apical dental abscesses were noted, suggesting an odontogenic origin (Figure ).
Figure 1. Case 1. (A) Preoperative CT scan—axial cut—demonstrating extensive soft tissue gas involving the neck and chest wall. (B) Preoperative examination with erythema and swelling involving the neck and chest. (C) Preoperative airway evaluation with edema and airway compromise. (D) Patient after first debridement and tracheostomy.
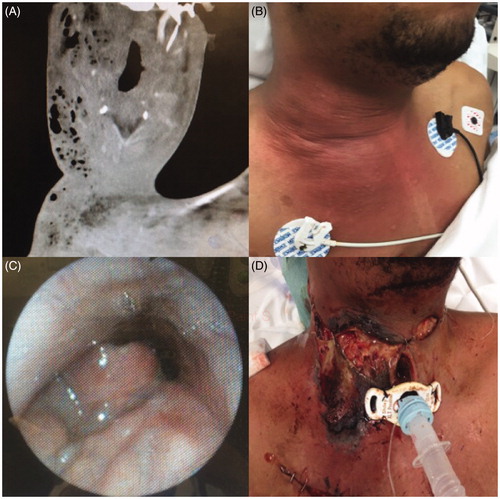
The patient subsequently developed worsening pharyngeal edema and airway compromise and was taken emergently to the operating room for tracheostomy and debridement (Figure ). He was found to have necrotic debris in the bilateral deep neck spaces and right chest soft tissue superficial to the pectoralis major muscle, as well as in the bilateral parapharyngeal spaces and right infratemporal fossa (Figure ). The patient was treated with IV vancomycin, metronidazole, and fluconazole during his hospitalization. Wound and tissue cultures grew streptococcus constellatus, streptococcus anginosis, and candida albicans. Blood cultures did not grow any organisms. No hyperbaric oxygen treatment was used.
Over the following 9 days, the patient underwent four additional surgical debridements. On hospital day 17, his wounds were deemed clinically free of infection and he was taken to the operating room for an anterolateral thigh (ALT) free flap reconstruction for his extensive thoraco-cervico-facial soft tissue defect and great vessel exposure (Figure ). Given the significant vessel depletion of his bilateral neck from prior infection and debridement, microvascular anastomoses were performed to the internal mammary vessels (Figure ). No intraoperative anticoagulation was provided. The patient received aspirin 325 mg orally for 5 days post-operatively.
Figure 2. Case 1. (A) Extensive thoraco-cervico-facial soft tissue defect and great vessel exposure following multiple debridements. (B) Immediate postoperative appearance following reconstruction with an anterolateral thigh free flap. (C) Patient appearance at follow-up.
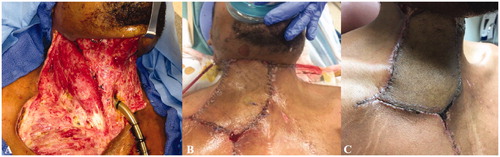
The patient recovered well post-operatively. He was decannulated, tolerated an oral diet, and had no perioperative complications. He was discharged home on postoperative day 4 from free flap reconstruction (hospital day 21). He returned to work approximately 2 months after surgery, and he had full range of motion in his neck at his last follow-up 8 months after his reconstruction (Figure ).
Case 2
A 67-year-old male with a past medical history of chronic obstructive pulmonary disease and congestive heart failure presented with acutely worsening neck swelling and airway compromise. He had no history of immunosuppression, diabetes, IV drug use, or other risk factors for severe necrotizing infection. Home medications included only simvastatin and lisinopril. Imaging was notable for an 8-cm retropharyngeal abscess extending into the deep spaces of the left neck with soft tissue gas extending to the anterior mediastinum (Figure ).
Figure 3. Case 2. (A) Pre-operative CT scan—axial cut—demonstrating extensive soft tissue gas involving the bilateral neck with extension into the anterior mediastinum. (B) Extensive soft tissue defect involving bilateral neck with exposure of larynx and great vessels.
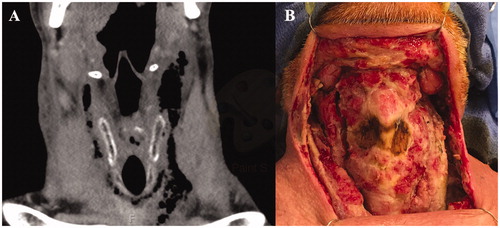
The patient underwent extensive debridement of the bilateral deep neck spaces and anterior cervical skin. Over the following 48 h, he underwent two additional debridements. The patient was treated with IV vancomycin and piperacillin–tazobactam during his hospitalization. Wound and tissue cultures grew mixed anaerobes. Blood cultures did not grow any organisms. No hyperbaric oxygen treatment was used.
On hospital day 8, the patient’s wounds were deemed clinically free of infection and he underwent ALT free flap reconstruction for his extensive bilateral neck soft tissue defect and exposed great vessels and larynx (Figure ). Adequate donor vessels were identified in the neck and microvascular anastomoses were performed to the facial artery and a ranine vein (Figure ). No intraoperative anticoagulation was provided. The patient received aspirin 325 mg orally for 5 days post-operatively.
Figure 4. Case 2. Reconstruction with an anterolateral thigh free flap. (A) Immediate postoperatively and (B) at follow-up.
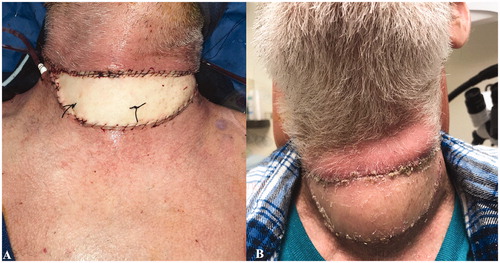
The patient recovered well and was discharged to a rehabilitation facility on postoperative day 6 (hospital day 14). He was noted to be doing well with a well-healing flap at his follow up visits 2 weeks and 1 month after discharge (Figure ), and he is currently living at home.
Discussion
The superficial cervical fascia and the superficial layer of the deep cervical fascia provide barriers against deep penetration of infection. Spread of necrotizing fasciitis through these layers may lead to loss of platysma and overlying skin, but frequently spares deeper critical structures. Through spread within the deep cervical fascia, those deeper critical structures may be lost and infection may gain access to the mediastinum, which is associated with high mortality [Citation8, Citation11]. With early recognition of the serious nature of the disease and aggressive surgical debridement, patients may survive. In surviving patients, the resulting soft tissue defect can usually be managed with primary closure or skin grafting [Citation9,Citation12].
However, in rare cases, the resulting surgical defect is so extensive that it may require vascularized reconstruction to provide tissue augmentation, minimize scar contraction, and obtain coverage of the great vessels. Free tissue transfer for extensive oncologic resection is well described and has provided a greater ability to perform tailored single-stage complex head and neck reconstruction. Specific challenges, however, arise in the setting of necrotizing fasciitis. The infection may lead to thrombosis of vessels frequently used for microvascular anastomosis, thus leading to a vessel-depleted neck. Furthermore, the same comorbidities associated with the development of necrotizing fasciitis are associated with free flap failure [Citation4].
There is very little data on the safety of microvascular reconstruction in necrotizing fasciitis, and most studies involve extremity reconstruction. In the largest series to date, Gawaziuk et al. reported on 12 patients undergoing free tissue transfer reconstruction among 224 patients with necrotizing fasciitis [Citation9]. The majority of patients were reconstructed for extremity defects at a mean of 11.6 days after initial admission. The most commonly used flap was ALT (n = 10), followed by latissimus dorsi (n = 1) and radial forarm (n = 1). There were no flap failures or complications requiring return to the operating room.
While these data demonstrates that free tissue transfer may be a safe and viable option for reconstruction for necrotizing fasciitis, reconstruction in the head and neck provides unique challenges. Involvement of deep neck lymphatic compartments can lead to inflammation of neck donor vessels potentially increasing the risk of vasospasm or vessel thrombosis. These challenges may then require use of vessels from outside of the neck, such as the internal mammary or thoracoacromial vessels.
To date, data demonstrating the feasibility of head and neck free flap reconstruction following cervicofacial necrotizing fasciitis is limited to single case reports. Of the cases described by Gawaziuk et al., only one flap involved reconstruction following cervicofacial necrotizing fasciitis, with a 15 × 15 cm defect reconstructed with a latissimus dorsi free flap [Citation9]. Additionally, Sykes et al reported on a case of massive cervicofacial necrotizing fasciitis that resulted in a right lower lip defect with exposed mandible and loss of major portions of the anterior neck and upper chest skin [Citation10]. The lower lip was reconstructed with an Estlander flap, and the neck skin with a scapular/parascapular bilobed skin paddle anastomosed to the internal jugular vein and the external carotid. The remaining chest skin was reconstructed with autologous skin graft.
The current report demonstrates two additional cases of reconstruction of extensive cervical defects following debridement for necrotizing fasciitis using ALT free flaps, the first case with exposed great vessels and the second with exposed great vessels and airway. These cases demonstrate the safety of using in-field neck vessels for microvascular anastomosis when available and the utility of extending to the internal mammary donor site when no neck vessels are suitable. In each case, the patient recovered well with a viable flap. While such cases are rare, with specific needs for free flap reconstruction, they further demonstrate that in select patients with extensive tissue defects and exposed critical structures from cervicofacial necrotizing fasciitis, free tissue transfer may be a safe and viable option.
Conclusion
In patients surviving extensive cervical necrotizing fasciitis, there may be a need for advanced reconstruction. While free tissue transfer may provide the ideal reconstructive option in such patients, it comes with risk of microvascular complications in a previously infected and widely debrided surgical bed. In appropriately selected patients, however, free flap reconstruction is both safe and feasible with use of either suitable in-field cervical donor vessels or internal mammary vessels in when necessary.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bahu SJ, Shibuya TY, Meleca RJ, et al. Craniocervical necrotizing fasciitis: an 11-year experience. Otolaryngol Head Neck Surg. 2001;125:245–252.
- Krespi YP, Lawson W, Blaugrund SM, et al. Massive necrotizing infections of the neck. Head Neck Surg. 1981;3:475–481.
- Bernal NP, Latenser BA, Born JM, et al. Trends in 393 necrotizing acute soft tissue infection patients 2000-2008. Burns. 2012;38:252–260.
- Tunovic E, Gawaziuk J, Bzura T, et al. Necrotizing fasciitis: a six-year experience. J Burn Care Res. 2012;33:93–100.
- Quereshy FA, Baskin J, Barbu AM, et al. Report of a case of cervicothoracic necrotizing fasciitis along with a current review of reported cases. J Oral Maxillofac Surg. 2009;67:419–423.
- Subhashraj K, Jayakumar N, Ravindran C. Cervical necrotizing fasciitis: an unusual sequel of odontogenic infection. Med Oral Patol Oral Cir Bucal. 2008;13:E788–91.
- Feinerman IL, Tan HK, Roberson DW, et al. Necrotizing fasciitis of the pharynx following adenotonsillectomy. Int J Pediatr Otorhinolaryngol. 1999;48:1–7.
- Sarna T, Sengupta T, Miloro M, et al. Cervical necrotizing fasciitis with descending mediastinitis: literature review and case report. J Oral Maxillofac Surg. 2012;70:1342–1350.
- Gawaziuk JP, Liu T, Sigurdson L, et al. Free tissue transfer for necrotizing fasciitis reconstruction: a case series. Burns. 2017;43:1561–1566.
- Whetzel TP, Sykes JM, Reilly DA. Acute reconstruction of massive cervicofacial necrotizing fasciitis with Estlander and free scapular/parascapular flaps. Otolaryngol Head Neck Surg. 1999;120:101–104.
- Mathieu D, Neviere R, Teillon C, et al. Cervical necrotizing fasciitis: clinical manifestations and management. Clin Infect Dis. 1995;21:51–56.
- Wagstaff MJ, Caplash Y, Greenwood JE. Reconstruction of an anterior cervical necrotizing fasciitis defect using a biodegradable polyurethane dermal substitute. Eplasty 2017;17:e3.
