Abstract
Interdigitating dendritic cell sarcoma (IDCS) is an extremely rare neoplasm derived from antigen-presenting cells of the immune system. It mostly occurs in the lymph node of the neck or axilla. We report a case of IDCS occurring in an 82-year-old female who presented with multiple masses in her right parotid gland. The patient was successfully treated with conservative surgery preserving the facial nerve followed by radiotherapy for the macroscopic lesion remnants. Most localized diseases were treated by surgery with or without irradiation, while advanced diseases were treated with systematic chemotherapy, such as CHOP. Radiotherapy may be an effective alternative to complete resection in patients with localized IDCS that involves functional and/or vital structures. The present case demonstrated that conservative surgery preserving the facial nerve followed by radiotherapy is an effective alternative option for the treatment of IDCS.
Introduction
Interdigitating dendritic cell sarcoma (IDCS) is an exceedingly rare neoplasm derived from antigen-presenting cells of the immune system. It mostly occurs in the cervical and axillary lymph nodes. To our knowledge, to date, only three patients with IDCS arising from the parotid gland has been reported. Most patients were treated with surgery with or without radiotherapy and/or chemotherapy, and prognosis varied.
Case report
An 82-year-old woman presented at our hospital with one month-long history of painful masses in her right parotid gland without other symptoms. She had no history of smoking or alcohol consumption. On physical examination, two elastic, hard, and mobile masses in her right parotid gland; one was in the upper pole and the other was in the lower pole of the parotid gland. Facial nerve paralysis was not observed and no remarkable findings were observed in ears, nose, oral cavity, and laryngopharynx. Compute tomography (CT) showed three solid masses in the parotid gland (Figure ). No malignant findings were obtained from fine needle aspiration cytology from the mass of the lower end. Based on these findings, we suspected Warthin’s tumor and decided to follow up with the patient’s wishes carefully. However, the mass in the lower pole of the parotid gland had enlarged over the course of 3 months after her first visit. Magnetic resonance imaging (MRI) showed enlarged masses in the parotid gland and lymphadenopathy in Level IIA (Figure ). Since the malignant tumor was suspected from the clinical course, we initially planned total parotidectomy with facial nerve preservation and modified radical neck dissection. However, the upper tumor invaded the zygomatic branch (Figure ) and the lower tumor was very close to the mandibular branch. Intraoperative frozen section of the lymph node of level IIA suggested the non-epithelial malignant tumor. Considering these findings, we decided to enucleate the tumor in the lower end and to resect the tumor in the upper-end part.
Figure 1. Contrast-enhanced CT findings at first visit and MRI findings 3 months after first visit. Contrast-enhanced CT at first visit showed one tumor in the upper end (A, arrowhead) and two tumors in the lower end (B, arrowhead) of parotid gland. MRI findings 3 months after the first visit showed enlarged tumors in axial section of T1 weighted intensity (C,D) and coronal section of T2 weighted intensity (E,F). Arrowheads indicate the tumors in the upper end (C,E) and the lower end (D,F) of parotid gland and superior deep lateral cervical lymph node (F).
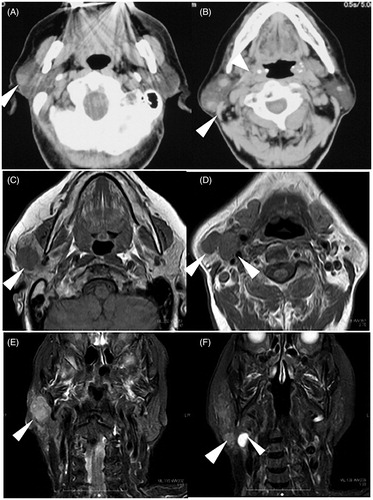
Figure 2. Intraoperative finding and resected specimen. Arrowheads indicate the main trunk of the facial nerve at the cross point (A). The upper end of tumor (white dotted circle) invaded the zygomatic branch of facial nerve. The surgical specimens were whitish in color (B).
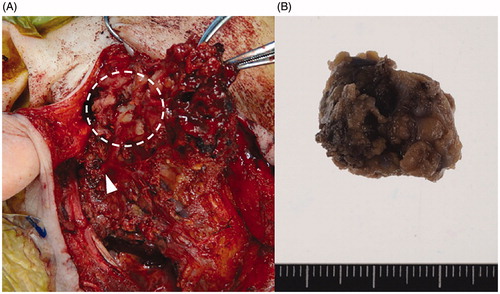
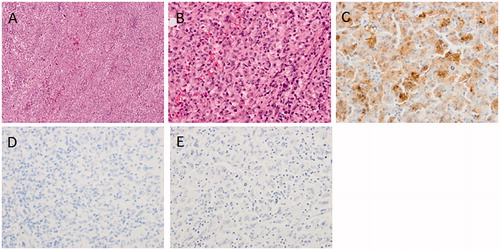
The surgical specimens were whitish in color and soft (Figure ). Microscopic examination of the tumor specimens from the parotid gland revealed that large clear cells propagated between neutrophils and lymphocytes. Tumor cells contained large nuclei with unique Notch complexes. The findings from the specimen of the lymph node were similar, but increased nuclear pleomorphism with unusual giant cells, and brisk mitotic activity were observed. Immunohistochemical studies showed diffuse positivity for S-100, CD163, and CD4. The specimen tested negative for all other markers: CD1a, CD21, CD30, HMB45, AE1/3, αSMA, and langerin (Figure ). The positivity of S-100, CD163, and CD4 indicated that the tumor was a histiocytic/dendritic cell tumor. The negativity of CD21 and CD23 did not correspond to a diagnosis of follicular dendritic cell sarcoma, whereas the negativity of CD1a and langerin did not correspond to a diagnosis of Langerhans cell sarcoma. Based on these finding, the final pathological diagnosis of IDCS was made.
Figure 3. Microscopic finding of resected tumor. (A) Hematoxylin and Eosin (HE) stained finding in low power field. (B) HE stained finding in high power field. (C) S-100 immunostained finding. (D) Langerin immunostained finding. (E) CD21 immunostained finding.
F-18-fluorodeoxyglucose (FDG) positron emission tomography (PET) showed no abnormal uptake in any other organs. A definite diagnosis of localized IDCS was made. Since the patient refused the radical operation with sacrificing the facial nerve, she received radiotherapy from 42 days after the surgery. The cumulative dose was 60 Gy per 30 fractions targeting the right parotid gland and right neck. Facial nerve paralysis was not observed during the treatment period. The tumor remnants decreased gradually after irradiation, and she finally achieved complete remission. She has been alive without disease for three years after completion of treatment.
Discussion
IDCS is an exceedingly rare neoplasm derived from antigen-presenting cells that activate T cells [Citation1]. The reported median age at diagnosis is 56.5 years with a slightly male dominant (M: F 1.38:1) and Asian descent predilection [Citation2]. The lymph node is the most common site for IDCS [Citation2]. However, this neoplasm has been described in extranodal sites, including the liver, skin, lungs, and gastrointestinal tract [Citation3,Citation4]. To date, there were only 4 cases of IDCS in the parotid gland, including the present case (Table ) [Citation5–8]. The majority of previously reported cases included the manifestation of a painless lump, but systemic symptoms such as fever, weight loss, and fatigue are not typical [Citation2]. The prognosis depends on whether the disease is metastatic. The 1- and 2-year survival rates for a local disease are 84.8% and 68.1%, respectively. However, for metastatic disease at onset, the rates were 38.5% and 15.8%, respectively [Citation2]. Most cases of local disease progress slowly, but several cases died of IDCS after progressing dramatically over several months from onset [Citation3,Citation4].
Table 1. Reported cases of IDCS of the parotid gland.
Histological features of IDCS are characterized by pleomorphic spindle cells with whorled and storiform patterns [Citation8]. Immunohistochemically, they showed consistent expression of S-100 and vimentin similar to those observed in Langerhans cell histiocytosis (LCH) and follicular dendritic cell sarcoma (FDCS). LCH was positive for CD1a and langerin, whereas some IDCS reveals consistent expression of CD1a, and FDCS expresses CD21 and CD23 [Citation1,Citation9]. A consensus on the treatment for IDCS has yet to be reached. In most cases, resectable localized ICDS is treated with surgery. Adjuvant radiotherapy and/or systemic chemotherapy were administered in several cases. Regimens commonly used in lymphomas, including CHOP (cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisolone), ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine), or ICE (ifosfamide, cyclosporin, and etoposide) has been reported, but their effect is not defined precisely [Citation2,Citation8].
In the present case, a Warthin’s tumor was suspected initially. As the tumor progressed rapidly, it was suspected to be malignant, and the complete surgical resection was scheduled. Accordingly, despite the invasion of the facial nerve, we performed the partial resection of the tumor to preserve the facial nerve and selected radiotherapy instead of radical surgery for the macroscopic lesion remnants. Finally, she achieved complete remission and has been alive without disease for three years. These results suggest that radiotherapy is an effective alternative option for the treatment of localized IDCS, although the role of radiation therapy for IDCS is not clearly defined as mentioned previously.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- O’Malley DP, Zuckerberg L, Smith LB, et al. The genetics of interdigitating dendritic cell sarcoma share some changes with Langerhans cell histiocytosis in select cases. Ann Diagn Pathol. 2014;18:18–20.
- Saygin C, Uzunaslan D, Ozguroglu M, et al. Dendritic cell sarcoma: a pooled analysis including 462 cases with presentation of our case series. Crit Rev Oncol Hematol. 2013;88:253–271.
- Pas TD, Spitaleri G, Pruneri G, et al. Dendritic cell sarcoma: an analytic overview of the literature and presentation of original five cases. Crit Rev Oncol Hematol. 2008;65:1–7.
- Sibutani M, Taraoka H, Nakao S, et al. A case of interdigitating cell sarcoma of the rectum. Jpn J Gastroenterol Surg. 2010;43:857–862.
- Lupato V, Romeo S, Franchi A, et al. Head and neck extranodal interdigitating dendritic cell sarcoma: case report and review of the literature. Head Neck Pathol. 2016;10:145–151.
- Barwell N, Howatson R, Jackson R, et al. Interdigitating dendritic cell sarcoma of salivary gland associated lymphoid tissue not associated with HHV-8 or EBV infection. J Clin Pathol. 2004;57:87–89.
- Sharma M, Ahsan F, Ah-See KW, et al. Interdigitating dendritic cell sarcoma of the parotid gland. J Laryngol Otol. 2006;120:244–246.
- Efune G, Sumer BD, Sarode VR, et al. Interdigitating dendritic cell sarcoma of the parotid gland: case report and literature review. Am J Otolaryngol. 2009;30:264–268.
- Gaertner EM, Tsokos M, Derringer GA, et al. Interdigitating dendritic cell sarcoma. A report of four cases and review of the literature. Am J Clin Pathol. 2001;115:589–597.
