Abstract
Conventional cochlear implantation (CI) is challenging in patients with coincidental anatomical or inflammatory middle ear pathologies. Performing subtotal petrosectomy (STP) simultaneously with CI allows to effectively overcome aforementioned problems. The aim of this study is to describe the benefits of simultaneous STP for our CI patients, review its indications, and analyze short-term outcomes. We describe five cases of successful CI using STP method, the indications for operation were advanced otosclerosis, vestibular schwannoma, chronic mastoiditis, cholesteatoma and previous implant bed infection. Five patients had a follow-up of 7–16 months. We had minor complications, which did not require medical intervention. We provide analysis of surgical strategy, postoperative complications and auditory performance of those patients. In this study, we found clear advantages of STP for our selected CI candidates. These were better exposure in cases with modified anatomy, radical eradication of the underlying disease and reduction of the secondary infection risk.
Introduction
Subtotal petrosectomy (STP) is partial exenteration of the temporal bone, which consists of blind sac closure of external acoustic canal (EAC), canal wall down mastoidectomy with complete removal of EAC skin, tympanic membrane and middle ear mucosa and/or disease, exenteration of the mastoid cells, removal of tympanic bone and, finally, obliteration of the surgical cavity with abdominal fat [Citation1]. There are four most important indications for STP: (a) elimination of recurrent infection by drilling out all air cell tracts and removal of large cholesteatomas; (b) removal of large tumors without intradural extensions; (c) isolation of the middle ear and mastoid from external environment to prevent intracranial spread of infection due to exposed dura and inner ear fluids; (d) allowing cochlear implantation (CI) in difficult cases [Citation1].
The importance of simultaneous STP in CI has been highlighted in recent literature [Citation1–6]. Candidates for CI may coincidentally have various middle ear inflammatory pathologies or challenging anatomical conditions, which make conventional CI very difficult to undertake. Previously, different techniques have been proposed to overcome aforementioned problems in candidates for CI [Citation7–11]. For instance, canal wall down mastoidectomy has been used in combination with CI for patients with chronic otitis media with or without cholesteatoma [Citation12]. Middle fossa approach has been used to bypass the middle ear cavity [Citation11]. However, all of those methods carry potential risks for post-implantation complications, ranging from electrode extrusion to life-threatening secondary postoperative meningitis [Citation4,Citation13]. Performing STP in combination with CI allows to avoid aforementioned problems, because it provides wide exposure of anatomical landmarks, enables definitive eradication of underlying disease and leads to full isolation of surgical cavity from the environment. The latter significantly reduces potential for infectious complications [Citation3].
The aim of this study was to describe the benefits of simultaneous STP for our CI patients, review its indications, and analyze short-term outcomes.
Patients and methods
This retrospective study was conducted following the principles stated in the Declaration of Helsinki. We describe five cases of patients who underwent CI with simultaneous STP at the Department of Otorhinolaryngology at our clinic between July 2017 and December 2018. Mean age of the patients was 52.8 years (range 1–75 years), three males and two females. Mean follow-up was 11.4 months (range, 7–16 months). Data collection included clinical indications, imaging findings, surgical features and postoperative complications. All patients underwent otological examination and preoperative audiological assessment, including pure-tone average (PTA; average of 0.5–1–2–4 kHz) and word recognition test (WRS). All patients underwent both gadolinium-enhanced magnetic resonance imaging (MRI) and high resolution computed tomography (HRCT) of the temporal bone. This study was performed with the approval of the Ethics Committee.
Results
Patients’ demographic and clinical data are summarized in Table . The profile of used cochlear implants is summarized in Table .
Table 1. Demographic and clinical data of the patients.
Table 2. Cochlear implant profile.
Description of cases
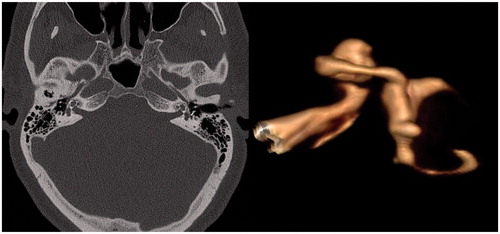
Patient 1 was referred to our department with bilateral profound hearing loss due to advanced otosclerosis. He has previously undergone multiple stapedoplasties on the left side. He had been using hearing aids in both ears, but speech discrimination became unsatisfactory during last years. HRCT scan revealed otosclerotic lesions bilaterally and ossification of the basal turn (BT) of both cochleas (Figure ). CI with simultaneous STP was undertaken on his left side for hearing rehabilitation. Left ear was selected due to more severe impairment of hearing. STP was considered in order to get full exposure of cochlea, to determine the course of its BT and to locate round window (RW) in an attempt to perform cochlear drill out and to insert the electrode into scala tympani (ST). However, RW was found to be ossified intraoperatively. After drilling in the area of expected RW, the ST was identified. The latter was followed anteriorly and was found to be obliterated. Therefore, opening of the scala vestibuli (SV) using separate cochleostomy was undertaken and electrode array was inserted into the latter. Postoperatively, patient developed mild disequilibrium which resolved spontaneously within 3 d. There were no other complications and follow-up has been uneventful so far. At the moment, patient uses CI on the left and conventional hearing aid on the right with WRS 81% at the level of 60 dB.
Figure 1. (A) HRCT showing bilateral cochlear otospongiosis and so-called halo effect (arrows). (B) 3 D reconstruction of MRI images shows complete obliteration of the basal turn of the scala tympani, but patent lumen of the scala vestibuli. That finding was confirmed intraoperatively.
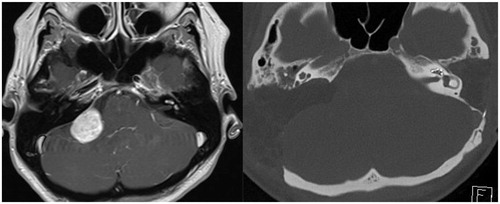
Patient 2 was referred to our department with intracochlear schwannoma on the left side and suspected vestibular schwannoma in the right cerebellopontine angle (CPA). She was suffering from bilateral sensorineural hearing loss (SNHL), tinnitus, dizziness and headaches for many years. By the time of admission, she had had profound hearing loss in her left ear for 7 years. She received radiotherapy (28 Gy) for the vestibular schwannoma at the right CPA 8 months earlier. After radiotherapy, her residual hearing on the right quickly deteriorated (Figure ). MRI T1 with gadolinium enhancement revealed two lesions: the first enhancing lesion located along the left BT of the cochlea and at the vestibule (indicating left intracochlear vestibular schwannoma) and the second enhancing mass positioned at the right CPA (Figure ). Our treatment and hearing rehabilitation plan consisted of two stages. First, we performed STP and cochlea drill-out on the left in order to remove intracochlear tumor and tumor from the vestibule. The intracochlear part of the tumor was found both in ST and vestibuli. Entire BT was opened, all tumor masses removed, taking care to preserve modiolus. Retrograde drilling of the SV opened vestibule and enabled to remove its tumor content. Surgery was continued by inserting electrode into ST of the remaining cochlea. The electrode array was sealed with temporal muscle fascia and covered with bone pate. Histopathological analysis confirmed the diagnosis of vestibular schwannoma. Nine months after the first surgery, translabyrinthine approach was undertaken to remove tumor from the right CPA. Histological investigation again showed vestibular schwannoma. Therefore, genetic testing of blood samples was undertaken to prove the diagnosis of neurofibromatosis 2 (NF2). However, the latter was not confirmed despite two separate set of genetic analyses. Postoperatively, the patient developed severe balance disorder due to bilateral complete loss of the vestibular function. The patient has been using CI for 4 months and by the time is only able to distinguish single sounds and can not understand speech without lipreading. Her WRS is as little as 11% at the level of 60 dB.
Figure 2. PTAs of the Patient 2 with intracochlear schwannoma in the left and vestibular schwannoma at the level of right CPA. (A) PTA before radiotherapy, (B) PTA 8 months after radiotherapy, showing quick deterioration of the hearing and development of almost complete deafness.
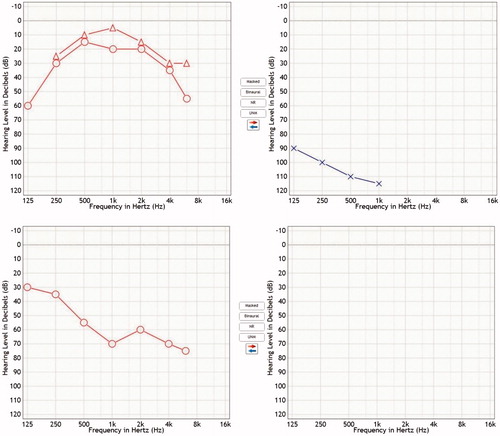
Figure 3. (A) Preoperative MRI T1 sequence with gadolinium enhancement showing tumor at the right CPA, but also contrasting small lesions along basal turn of the left cochlea and vestibule. (B) Postoperative CT scan showing electrode array in the left cochlea after subtotal petrosectomy and large craniotomy for translabyrinthine approach removal of tumor from the right.
Patient 3 was referred to our department with bilateral sensorineural deafness. He had previously undergone mastoidectomy on the right due to mastoiditis in another hospital, which resulted in complete deafness. He had also lost hearing from his left ear for unknown reason. HRCT scan showed typical post-mastoidectomy findings on the right. The treatment plan consisted of STP on the right for eradication of any inflammatory mastoid cells, followed by immediate CI. The operation was performed as a single-stage surgery because there was no active infection in the affected mastoid. Postoperatively, the patient developed small periocular hematoma which absorbed within a matter of days. Otherwise, the postoperative course was uneventful. At the moment, the patient is active user of CI and communicates effectively. His WRS is 70% at the level of 60 dB.
Patient 4 was admitted to our clinic with bilateral cholesteatoma and bilateral profound SNHL. Treatment plan consisted of bilateral eradication of the cholesteatoma using STP, followed by unilateral CI. During surgery, significant erosion of the BTs of both cochleas was observed with exposure of membranous labyrinth. The latter explains her complete bilateral deafness. The CI was performed on the left: the electrode was inserted into ST and cochlea was sealed and reconstructed with bone pate. Postoperatively, the patient experienced severe imbalance, vertigo and nausea. These symptoms relieved within 1 month. Patients’ postoperative WRS score is 12% at the level of 60 dB.
Patient 5 was 11 years old boy who was referred to our department for the reimplantation. He had undergone for bilateral CI 6 years prior to admission due to congenital profound bilateral SNHL. The right cochlear implant was removed 8 months after the first surgery due to infectious sequelae. He also experienced skin necrosis over the implant, which required excision of the necrosis and transposition skin flap. In order to prevent further infectious complications, reimplantation was performed via STP. Postoperatively, the patient developed hematoma of the abdominal wound, which absorbed spontaneously without any treatment. The first 7 months of follow-up have been otherwise uneventful. WRS with his right year is 0% at the level of 60 dB.
Discussion
CI is an acknowledged method for hearing rehabilitation in patients with severe to profound SNHL with relatively low complication rate [Citation2]. In difficult situations, such as malformed or ossified cochlea, middle ear or intralabyrinthine tumors, chronic suppurative otitis media (CSOM), cholesteatoma and radical cavity from previous surgeries and its postoperative secondary infections, it is recommended to perform simultaneous STP [Citation1–6]. This surgical approach provides an excellent exposure of the cochlea during CI and enables total isolation of surgical cavity from the outside environment, preventing secondary infectious complications. In this study, we describe clinical data and indications for STP as a part of CI in our first five patients, which are discussed in detail further.
Cochlear ossification
Far-advanced or retrofenestral forms of otosclerosis may cause profound SNHL by narrowing the cochlear lumen with distortion of the basilar membrane and by lytic enzymatic activity in the perilymph [Citation14]. Conventional CI in patients with severe SNHL as a result of otosclerosis may present a difficulty to the surgeon due to otospongiotic cochlea and possible obliteration of its lumen [Citation5]. The latter could make finding the RW and identifying the BT of the cochlea through narrow posterior tympanotomy very challenging. Undertaking STP in those cases enables easier identification of important landmarks, provides wide exposure of the entire cochlea, helps to locate the RW area and the BT. Moreover, in case of severe ossification of the cochlea, STP is the only method to provide adequate exposure needed for safe cochlear drill-out. Without good exposure, the latter can be dangerous due to close proximity of the cochlea to the internal carotid artery and the jugular bulb. In our patient with advanced otosclerosis, electrode insertion into ST was planned despite preoperative evidence of its obliteration on imaging. Therefore, STP was performed to get maximum exposure for cochlear drill-out. Intraoperatively, the decision was re-evaluated due to significant cochlear ossification and electrode was inserted into SV. In this case, benefits of STP were not fully executed because SV insertion could also have been achieved through facial recess during conventional CI as well.
Intracochlear tumors
Intralabyrinthine schwannomas are a rare subgroup of benign tumors of the 8th cranial nerve [Citation15]. Several strategies have been proposed to perform CI in those patients, including insertion of the electrode through the tumor without its removal [Citation16]. Application of such approach assumes that intracochlear tumor is slow-growing and its removal avoids the need for cochlear drill-out and will potentially give better hearing outcome. However, up to 15% of patients may show progressive growth of the intracochlear tumor [Citation17]. Therefore, we made a decision to remove the tumor prior to implantation. In addition, vestibular schwannomas in NF2 patients show more rapid tumor growth and aggressive course of disease compared to sporadic schwannomas [Citation18].
STP enabled full exposure of the cochlea and to perform safe drill-out procedure for intracochlear tumor removal. CI was simultaneously performed and electrode was inserted into partially drilled cochlea. That was the only way to provide hearing rehabilitation to this patient, because she rapidly lost hearing in the right ear after radiotherapy for vestibular schwannoma in the right CPA. Due to failure of radiotherapy and loss of hearing, the translabyrinthine approach was performed to remove schwannoma from the right CPA. Translabyrinthine approach was preferred due to unserviceable hearing and the size of the tumor (3 cm). Combining translabyrinthine approach with CI was not considered because cochlear nerve was not preserved. The patient has low WRS score probably as a consequence of too aggressive cochlea drill-out procedure. One of the possibilities to preserve more cochlea would have been pushing tumor out in a retrograde way from the medial turn. Another option to rehabilitate hearing is this patient is auditory brainstem implantation, because cochlear nerve is still intact in the left. However, CI was our first choice because literature shows its superiority over auditory brainstem implantation regarding hearing results [Citation19].
Chronic otitis media
Performing STP in situations with underlying CSOM and other associated problems (e.g. prior radical cavities, cholesteatoma and adhesive otitis) allows to obtain an aseptic field with no underlying disease, which is fundamental for favorable long-term outcomes in all implantable auditory devices including CI. We, therefore, chose to perform CI with simultaneous STP in our patients 3 and 4.
In patient 3, as there were several mastoid cells preserved, it carried a high risk to put the implant and the electrode into chronically inflamed area, which might have led to following labyrinthitis and meningitis.
In case 4, the patient with bilateral cholesteatoma, it was decided to perform STP simultaneously with CI. Though some recommend staging the surgery due to the risk of recurrence of cholesteatoma, it is not necessary in inactive cases where STP provides a wide exposure and enables definite removal of the disease. Radical removal of all mastoid cell tracts and underlying disease, as during STP, provides definitive solution and effectively prevents potential recurrences and multiple unnecessary reoperations [Citation2,Citation3]. Unfortunately, this patient gained very low WRS postoperatively. We suggest that bad performance of the cochlear implant, in this case, may be related to possible postoperative cochlear ossification due to its erosion by underlying disease.
Reimplantation after failed primary CI
In our 5th case, we described a deaf child who required reimplantation after removal of previous cochlear implant due to implant bed infection. The infection and explantation of the previous cochlear implant took place 8 months after CI, which indicates possible ascending infection. Therefore, the conventional cochlear reimplantation was not performed in fear of new infectious complications. Reimplantation was performed many years later in combination with STP. The latter leads to isolation of the mastoid cavity from the external environment and is the most effective way to prevent further ascending infections. The only downside of this case is that there was significant time gap between explantation and reimplantation, which has negative impact on the outcome of hearing, causing low WRS score.
Conclusions
In this study, we found clear advantages of STP for our selected CI candidates. Advantages of the STP include maximum exposure in cases of difficult anatomy, full and safe eradication of the underlying disease and minimizing possibility for secondary infectious complications. All our patients had different indications, including advanced otosclerosis and cochlear ossification, intracochlear schwannoma, CSOM, bilateral cholesteatoma with cochlear erosion and reimplantation after failed primary CI due to infection. We had minor complications, which did not require medical intervention. Longer follow-up is needed to confirm that STP is safe and efficient method for CI candidates with abovementioned problems.
Disclosure statement
The authors report no conflict of interests.
References
- Prasad S, Roustan V, Piras G, et al. Subtotal petrosectomy: surgical technique, indications, outcomes, and comprehensive review of literature. Laryngoscope. 2017;127(12):2833–2842.
- Altuna X, García L, Martínez Z, et al. The role of subtotal petrosectomy in cochlear implant recipients. Eur Arch Otorhinolaryngol. 2017;274(12):4149–4153.
- Polo R, Del Mar Medina M, Arístegui M, et al. Subtotal petrosectomy for cochlear implantation. Ann Otol Rhinol Laryngol. 2015;125(6):485–494.
- Free R, Falcioni M, Di Trapani G, et al. The role of subtotal petrosectomy in cochlear implant surgery–a report of 32 cases and review on indications. Otol Neurotol. 2013;34(6):1033–1040.
- Vashishth A, Fulcheri A, Prasad S, et al. Cochlear implantation in cochlear ossification. Otol Neurotol. 2017;39:17–28.
- Gao S, Jiang Y, Wang G, et al. Cochlear implantation in patients with canal wall down mastoidectomy cavities. Acta Oto-Laryngol. 2018;138(11):993–997.
- Kim C, Chang S, Lee H, et al. Cochlear implantation in patients with a history of chronic otitis media. Acta Oto-Laryngol. 2004;124(9):1033–1038.
- Manrique M, Cervera-Paz F, Espinosa J, et al. Cochlear implantation in radical cavities of mastoidectomy. Laryngoscope. 1996;106(12):1562–1565.
- Kojima H, Sakurai Y, Rikitake M, et al. Cochlear implantation in patients with chronic otitis media. Auris Nasus Larynx. 2010;37(4):415–421.
- Xenellis J, Nikolopoulos T, Marangoudakis P, et al. Cochlear implantation in atelectasis and chronic otitis media. Otol Neurotol. 2008;29(4):499–501.
- Colletti V, Fiorino F, Carner M, et al. New approach for cochlear implantation: cochleostomy through the middle fossa. Otolaryngol Head Neck Surg. 2000;123(4):467–474.
- Wong M, Shipp D, Nedzelski J, et al. Cochlear implantation in patients with chronic suppurative otitis media. Otol Neurotol. 2014;35(5):810–814.
- Lalwani A, Cohen N. Does meningitis after cochlear implantation remain a concern in 2011? Otol Neurotol. 2012;33(1):93–95.
- Rotteveel L, Proops D, Ramsden R, et al. Cochlear implantation in 53 patients with otosclerosis: demographics, computed tomographic scanning, surgery, and complications. Otol Neurotol. 2004;25(6):943–952.
- Plontke S, Rahne T, Pfister M, et al. Intralabyrinthine schwannomas. HNO. 2017;65(S2):136–148.
- Carlson M, Neff B, Sladen D, et al. Cochlear implantation in patients with intracochlear and intralabyrinthine schwannomas. Otol Neurotol. 2016;37(6):647–653.
- Salzman K, Childs A, Davidson H, et al. Intralabyrinthine schwannomas: imaging diagnosis and classification. Am J Neuroradiol. 2012;33(1):104–109.
- Kaul V, Cosetti M. Management of vestibular schwannoma (including NF2). Otolaryngol Clin North America. 2018;51(6):1193–1212.
- Medina M, Di Lella F, Di Trapani G, et al. Cochlear implantation versus auditory brainstem implantation in bilateral total deafness after head trauma. Otol Neurotol. 2014;35(2):260–270.
