Abstract
Benign nasal septal tumors are rare and exhibit various histologic features. It is necessary to treat them based on a plan that considers the prognosis, functionality, and esthetics. We report two similar and rare cases of nasal septal tumors with different diagnoses that we treated with nasal septal tumor resection. Eosinophilic angiocentric disease was the most likely diagnosis of one of the two cases, but it was not confirmed by the IgG4:IgG ratio. The other case was diagnosed as extranodal Rosai-Dorfman disease based on its histological features. The histological appearance of nasal septal tumors varies widely; therefore, a careful diagnosis based on both clinical and pathological perspectives is required.
Introduction
Benign nasal septal tumors are rare and exhibit various histologic features. We report two similar and rare cases of nasal septal tumors with different diagnoses treated with nasal septal tumor resection. The first case was diagnosed as eosinophilic angiocentric fibrosis-like disease, and the second was diagnosed as extranodal Rosai-Dorfman disease. Because the histological appearance of nasal septal tumors varies widely, a careful diagnosis based on both clinical and pathological perspectives is required.
Case presentation
Case 1
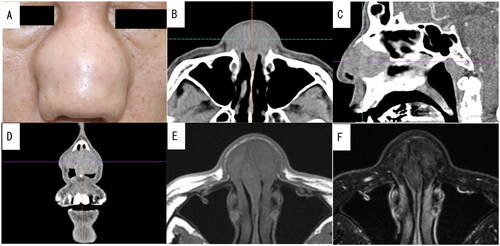
A 55-year-old Japanese man presented with a 1-year history of severe nasal swelling and obstruction. He had no other medical history. The nasal cavity was severely deformed and completely occluded by tumors, thus presenting a smooth surface covered with nasal mucosa. Enhanced computed tomography imaging of the nasal sinus revealed a slightly enhanced tumor measuring 36 × 33 mm that was situated in the nasal septum (Figure ). No evidence of invasion of the maxillary bone or distant metastasis was observed during the imaging studies. The laboratory results indicated a normal white blood cell level (8.80 × 103/μL), increased eosinophil ratio (13.5%), and increased immunoglobulin E level (588 mg/dL). The IgG and IgG4 levels were 1293 mg/dL and 32.1 mg/dL, respectively; therefore, they were within the normal ranges. To determine allergen-specific IgE levels, the patient was sensitized to house dust mites and Japanese cedar pollen using ImmunoCAP® testing (SRL Inc., Tokyo, Japan). Autoimmune screening tests for various antibodies, including anti-U1 ribonucleoprotein antibody, anti-Sjögren’s syndrome A antibody, anti-Sjögren’s syndrome B antibody, anti-myeloperoxidase antibody, anti-PR3 antibody, and antinuclear body, yielded negative results. The interferon-gamma release assay and fluorescent treponemal antibody test also yielded negative results. To confirm the diagnosis, an open excisional biopsy of a solid lesion in the left nasal cavity was performed. Microscopic examination of the biopsy specimen revealed infiltration of inflammatory cells, including neutrophils, lymphocytes, plasma cells, and eosinophils, and severe fibrosis. Although the lesion appeared to be benign, a definitive diagnosis could not be determined. Oral steroids were initiated at a dose of 30 mg daily to reduce inflammation; however, they were ineffective. Consequently, tumor resection was performed under general anesthesia.
Figure 1. Preoperative photograph of the patient and enhanced computed tomography (CT) and plain magnetic resonance imaging (MRI) images of the nasal sinus of case 1. A well-defined mass measuring 36 × 33 mm was visualized using contrast-enhanced CT without any contrast effect. It had replaced the nasal septal cartilage. Additionally, T1-weighted and T2-weighted MRI revealed a mass with low signal intensity. (A) Preoperative photograph showing swelling from the nasal bridge to the nasal wing. (B) Axial CT image. (C) Sagittal CT image. (D) Coronal CT image. (E) Axial MRI T1-weighted image. (F) Axial MRI T2-weighted image.
During open rhinoplasty, it was observed that the tumors consisted of three parts. One part was sandwiched between the nasal septal cartilage, and the other two parts gripped the major alar cartilages on both sides. Because of tumor involvement, the nasal septal cartilage, a portion of the nasal crest, and bilateral major alar cartilages were resected. Struts were harvested from the costal cartilage for reconstruction. The struts were modified into an L-shape and implanted between the nasal septal mucosa.
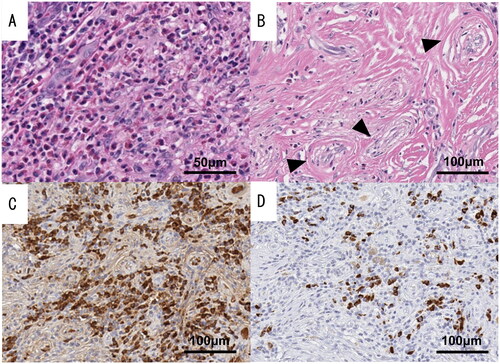
The specimens were yellowish-white and adhered to the cartilage. The microscopic examination showed that the resected tissue had inflammatory findings similar to those observed during the preoperative biopsy. The tissue predominantly comprised dense fibrosis with concentric, perivascular fibrosis, which is a characteristic feature of eosinophilic angiocentric fibrosis (EAF) known as onion skin fibrosis (Figure ). Although there were some IgG4-positive plasma cells in the tissue, the IgG4:IgG ratio in plasma cells was less than 40% (Figure ). Based on these findings, we concluded that this case was an EAF-like disease because it did not meet the diagnostic criteria for IgG4-related disease.
Figure 2. Pathological findings of resected specimens of case 1. The presence of onion skin fibrosis (black triangles) and clustered eosinophils supported the diagnosis of eosinophilic angiocentric fibrosis (EAF). However, the IgG4:IgG ratio in plasma cells was less than 40%. (A) Hematoxylin and eosin stain (high-power field). (B) Hematoxylin and eosin stain (low-power field). (C) IgG immunochemical stain. (D) IgG4 immunochemical stain.
At the 18-month postoperative follow-up examination, the patient’s nasal patency had improved, and no recurrence was noted. The cosmetic and functional outcomes were satisfactory.
Case 2
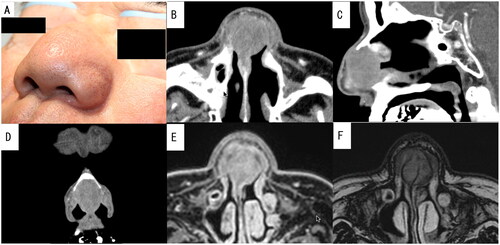
A 63-year-old Japanese man presented with a 2-year history of nasal obstruction and deformity. Approximately 30 years prior to presentation, he had undergone bilateral Caldwell-Luc surgery for chronic sinusitis. The patient denied any history of allergies. During the initial consultation, thickening of the nasal septum was observed, and tumorous lesions with a smooth surface occupied both nasal cavities, making it difficult to observe the posterior nasal cavities. Enhanced computed tomography revealed a mass measuring 29 × 28 mm that had replaced the nasal septal cartilage (Figure ). Except for a slightly increased C-reactive protein level, almost all hematologic parameters were within the normal ranges. The results of autoimmune screening tests, interferon-gamma release assays, and fluorescent treponemal antibody tests were also negative. A microscopic examination of the open excisional biopsy specimen showed infiltration of inflammatory cells, including plasma cells, eosinophils, and histiocyte-like foam cells, and severe fibrosis. Oral administration of steroids at a dose of 30 mg per day was initiated; however, the tumor size did not decrease.
Figure 3. Preoperative photograph of the patient and enhanced computed tomography (CT) and plain magnetic resonance imaging (MRI) images of the nasal sinus of case 2. A well-defined mass measuring 29 × 28 mm was visualized using contrast-enhanced CT without any contrast effect. It had replaced the nasal septal cartilage. Additionally, both T1-weighted and T2-weighted MRI showed a mass with low to normal signal intensity. (A) Preoperative photograph showing symmetrical swelling from the nasal dorsum to the nasal alae. (B) Axial CT image. (C) Sagittal CT image. (D) Coronal CT image. (E) Axial MRI T1-weighted image. (F) Axial MRI T2-weighted image.
Consequently, surgery was performed under general anesthesia. The tumor was resected via open rhinoplasty. Because the tumor was adhered to the nasal septal mucosa and septal cartilage, en bloc resection was performed, and the harvested costal cartilage was modified into an L-shape and used to reconstruct the defect. The surface of the costal cartilage was covered by rotating the nasal basal mucosa.
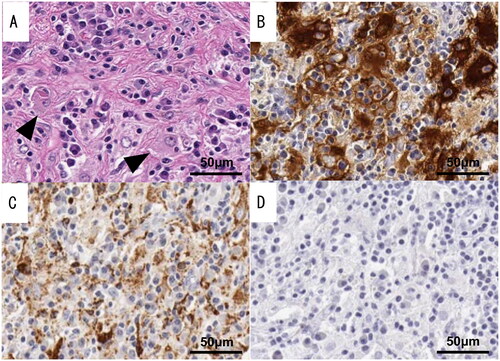
The histopathologic examination showed marked histiocytic infiltration accompanied by mononuclear cells, such as lymphocytes and plasma cells. Emperipolesis, a process by which one cell engulfs and temporarily retains another cell within its cytoplasm, has been observed in immune cells, particularly macrophages (Figure ). In the present study, a small number of histiocytes demonstrated emperipolesis. An immunohistochemical analysis revealed positive staining for S-100 protein and CD68 but negative staining for CD1a (Figure ). These pathological findings supported the diagnosis of extranodal Rosai-Dorfman disease (RDD). At 19 months after surgery, marked improvement in nasal congestion was observed. No postoperative treatment was administered.
Figure 4. Pathological findings of resected specimens of case 2. The tissue shows a histological picture consisting of histiocytic infiltration with foamy histiocytes and diverse inflammatory cell infiltration. Emperipolesis, a process by which one cell engulfs and temporarily retains another cell within its cytoplasm, has been observed in immune cells, particularly macrophages (black triangles). The immunohistochemical analysis revealed positive staining for S-100 protein and CD68 but negative staining for CD1a. (A) Marked histiocytic infiltration. Emperipolesis was confirmed in a few histiocytes. (B) Immunohistochemical staining for S-100 protein. (C) Immunohistochemical staining for CD68. (D) Immunohistochemical staining for CD1a.
Discussion
We managed two uncommon nasal septum diseases. Because several other conditions were listed as possible differential diagnoses, including antineutrophil cytoplasmic antibody-associated vasculitis, tuberculosis, and malignant tumors, various examinations, including an open excisional biopsy, were conducted. A conclusive diagnosis could not be determined prior to surgery. Because the histopathologic characteristics were infrequently reported, a definitive diagnosis was determined based on the characteristics of the resected specimens.
EAF was named by Roberts and McCann in 1985 based on its histopathologic features [Citation1]. It most often occurs in the upper respiratory tract and usually has slow growth. The histopathology of EAF is considered to include two disease progression states, the inflammatory state and fibrotic state, with no clear boundaries. The coexistence of both stages in the same specimen, as observed in our case, is observed in most cases. Whorled perivascular fibrosis, or onion skin fibrosis, is more prominent during the late stages and often increases the specificity of the diagnosis of EAF [Citation2]. To our knowledge, only 36 nasal septal EAF cases have been reported; therefore, nasal septal EAF is rare. The average age at diagnosis is 49.9 years (range, 19-79 years), with a male-to-female ratio of 20:16; furthermore, it most commonly occurs in Caucasian patients [Citation2,Citation3].
RDD was first reported by Rosai and Dorfman in 1969, who described it as sinus histiocytosis with massive lymphadenopathy [Citation4]. The most common presentation is painless massive cervical lymphadenopathy in children and young adults [Citation5]. Although it is fundamentally lymphadenopathy, Gaurav reported that 92% of patients had extranodal disease and 67% of patients had only extranodal disease. Extranodal involvement mostly occurs in the skin, subcutaneous tissue, bone, upper respiratory tract (including the sinonasal cavities), glandular tissue, and kidneys [Citation6]. The cause of RDD is unclear, but it can respond to infectious antigens (human herpesvirus-6, parvovirus B19, and Epstein-Barr virus) or immune antigens [Citation5]. Histiocyte phagocytosis of emperipolesis, lymphocytes, plasma cells, erythrocytes, or polymorphonuclear leukocytes is considered an important histopathologic feature, along with positive staining for S-100 and CD68 in tissue phagocytic cells [Citation7]. In contrast, extranodal RDD demonstrates more severe fibrosis and fewer histiocytes in the lesion [Citation7], suggesting the possibility of a misdiagnosis. Although there are similar diseases, immunochemical staining often helps determine the final diagnosis. To our knowledge, only 12 cases of nasal septal RDD have been reported (median age at diagnosis, 33.7 years; range, 10-64 years; only two patients also had lymphadenopathy). At least five cases were observed in Asian patients, and the slight male predominance (male-to-female ratio, 7:5) is possibly linked to the proportion of cutaneous RDD presentations [Citation4–7].
EAF and RDD share some characteristics. Both are slowly progressive fibroinflammatory diseases that can occur reactively in the nasal cavity. Pathological findings are required for the diagnosis. In 2011, Deshpande et al. found that patients with EAF had dramatically increased serum IgG4 levels [Citation8]. Since then, the infiltration of IgG4-positive plasma cells and a high IgG4:IgG ratio have been frequently reported among patients with EAF [Citation2]. Although there is some literature suggesting that RDD in case 2 is similar to IgG4-related disease [Citation7], the overwhelming majority of cases are now referred to as mimickers of IgG4-related disease and are no longer discussed as a group of IgG4-related diseases [Citation9]. RDD is treated as a formal exclusion criterion for the diagnosis of IgG4-related disease [Citation10].
The cause of IgG4-related disease remains elusive. This disorder is generally characterized by synchronous or metachronous swelling, nodules, and hypertrophic lesions in diverse organs throughout the body that are attributable to the pronounced infiltration of lymphocytes, including IgG4-positive plasma cells, as well as fibrosis and increased serum IgG4 levels. In accordance with the comprehensive diagnostic criteria for IgG4-related disease [Citation10], from a pathological perspective, the number of IgG4-positive plasma cells should exceed 40 per high-power field, and the IgG4:IgG ratio should be ≥40%. Affected organs may include the pancreas, lacrimal glands, and salivary glands. Although the disease often involves multiple lesions, it may present as a single-organ lesion. The blood IgG4 level of case 1 described in this report was below the normal range. The pathological examination of case 1 confirmed that the IgG4:IgG ratio of the plasma cells of the resected specimen was less than 40%. Although the diagnostic criteria for IgG4-related disease were not met and a definitive diagnosis of EAF could not be made, the histological and clinical presentations of case 1 were similar to those of EAF. Notably, although case 2 had a clinical presentation similar to that of case 1, the two cases were found to be different diseases based on the clustering of tissue classes observed during the microscopic evaluation. The differential diagnosis of nasal septal tumors is broad and often involves the consideration of IgG4-related disease. Although a definitive diagnosis was not determined for case 1, we believe that it is valuable to share the diagnostic process.
Conclusion
We encountered two rare diseases that affected the nasal septum. They were successfully treated using open rhinoplasty with nasal septum reconstruction. Our findings underscore the significance of a comprehensive approach to the differential diagnosis when managing uncommon conditions that manifest with comparable clinical features. Furthermore, they emphasize the essential role of a meticulous pathological evaluation in establishing an accurate diagnosis.
Consent form
Both patients provided written informed consent for publication.
Acknowledgements
We express special thanks to the staff of the Department of Otolaryngology, Head and Neck Surgery, at Chiba University. Their advice was essential to the definitive diagnosis and treatment of this case. I express my great appreciation to the staff of the Pathology Department at Chiba University for providing essential data for the definitive diagnosis and reviewing the literature with me.
Disclosure statement
The authors report there are no competing interests to declare.
References
- Roberts PF, McCann BG. Eosinophilic angiocentric fibrosis of the upper respiratory tract: a mucosal variant of granuloma faciale? A report of three cases. Histopathology. 1985;9(11):1217–1225. doi: 10.1111/j.1365-2559.1985.tb02801.x.
- Chew EJC, Lee M-HH, Chung H-W, et al. Eosinophilic angiocentric fibrosis and immunoglobulin 4-related disease revisited. Histopathology. 2022;81(2):149–158. doi: 10.1111/his.14646.
- Jain R, Robblee JV, O’Sullivan-Mejia E, et al. Sinonasal eosinophilic angiocentric fibrosis: a report of four cases and review of literature. Head Neck Pathol. 2008;2(4):309–315. doi: 10.1007/s12105-008-0077-y.
- Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63–70.
- Azari-Yaam A, Abdolsalehi MR, Vasei M, et al. Rosai-Dorfman disease: a rare clinicopathological presentation and review of the literature. Head Neck Pathol. 2021;15(1):352–360. doi: 10.1007/s12105-020-01183-7.
- Goyal G, Ravindran A, Young JR, et al. Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica. 2020;105(2):348–357. doi: 10.3324/haematol.2019.219626.
- Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. 2020;73(11):697–705. doi: 10.1136/jclinpath-2020-206733.
- Deshpande V, Khosroshahi A, Nielsen GP, et al. Eosinophilic angiocentric fibrosis is a form of igG4-related systemic disease. Am J Surg Pathol. 2011;35(5):701–706. doi: 10.1097/PAS.0b013e318213889e.
- Chen LYC, Mattman A, Seidman MA, et al. IgG4-related disease: what a hematologist needs to know. Haematologica. 2019;104(3):444–455. doi: 10.3324/haematol.2018.205526.
- Wallace ZS, Naden RP, Chari S, et al. The 2019 American College of rheumatology/European league against rheumatism classification criteria for IgG4-related disease. Arthritis Rheumatol. 2020;72(1):7–19. doi: 10.1002/art.41120.
