Abstract
Background
Fungal rhinosinusitis is subdivided into two subtypes, invasive and noninvasive variants, with allergic fungal rhinosinusitis (AFRS) being the most prevalent noninvasive variant. The most common clinical features of AFRS are nasal polyposis, rhinorrhea, facial pressure, and periorbital edema.
Objective
This study aimed to report a rare clinical presentation and serious complications of AFRS.
Method and result
The patient was presented to the Otorhinolaryngology Department of Qatif Central Hospital, Eastern Region, Saudi Arabia. We reported a rare presentation of a patient diagnosed with untreated AFRS complicated by basilar artery thrombosis. The patient was urgently managed with endoscopic sinus surgery, postoperative antifungal, and local nasal steroids.
Conclusion
Allergic fungal rhinosinusitis is a chronic disorder associated with significant morbidity that requires early diagnosis and management. The treatment protocol was based on surgical debridement of the paranasal sinuses from the fungal material and postoperative steroid therapy. Allergic fungal sinusitis with granuloma was more aggressive and needed to be treated cautiously by adding antifungal treatments.
Introduction
Fungal rhinosinusitis is classified into invasive and noninvasive types, which describe whether the fungi have invaded the tissues. Allergic fungal rhinosinusitis (AFRS) is a subtype of chronic rhinosinusitis that is associated with inflammatory and allergic reactions to fungal antigens in the sinonasal tract. Moreover, it was first described in 1983 [Citation1]. Approximately 1–2% of the global population is affected by AFRS [Citation2]. There is a variety of incidences among different regions of the world, as AFRS is most prevalent in warm and humid climates such as India, the Middle East, and the southeastern United States [Citation3]. AFRS is diagnosed in immunocompetent patients with chronic rhinosinusitis symptoms and who meet the following Bent and Khun criteria: 1. Type I hypersensitivity; 2. Nasal polyposis; 3. Characteristic computed tomography (CT) findings; and 4. Eosinophilic mucin without invasion; and 5. Positive fungal stain [Citation4]. A definitive diagnosis of invasive fungal sinusitis was made by histopathological examination. The paranasal sinus mucosa shows necrosis owing to fungal vascular invasion, which causes thrombosis and infarction, leading to tissue destruction.
We report the case of an immunocompetent Indian male patient with a history of untreated chronic AFRS complicated by basilar artery infarction owing to the compression effect of the expanded sphenoid sinus disease.
Case presentation
The patient was a 48-year-old Indian male, not known to have any chronic medical illness. He was presented to the emergency department at Qatif Central Hospital, Eastern region, with a history of worsening headache and subjective fever associated with a decreased level of consciousness, in addition to a previous history of untreated chronic rhinosinusitis.
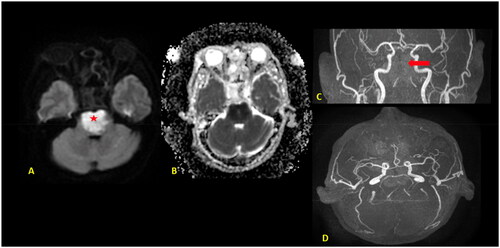
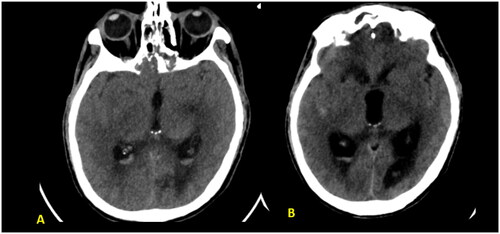
As the patient’s level of consciousness deteriorated, immediate intubation and an urgent CT scan of the brain were performed, which revealed pan-opacification of all the paranasal sinuses with expansion and bone remodeling, as well as a dense basilar artery (Figure ). The ENT team was consulted for a brain CT scan and sinusitis and basilar artery thrombosis were detected. Subsequently, Dedicated Magnetic Resonance Imaging of the brain and time-of-flight (TOF) MR angiography of the cerebral arteries were performed, confirming the presence of basilar artery occlusion with a resultant acute pontine infarction (Figure ).
Figure 1. (A, B) Unenhanced axial CT brain images revealed complete hyper-attenuating soft tissue opacification of the expanded sphenoid and ethmoid sinuses as well as visualized maxillary antra with sinus walls destruction and infiltration into cavernous sinus bilaterally and pre pontine cistern. Dense basilar artery indicating thrombosis (red arrow).
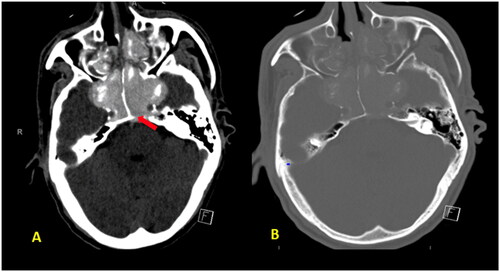
Figure 2. MRI Brain with axial diffusion restriction image (A) and apparent diffusion coefficient (ADC) (B) map shows an area of diffusion restriction in the pons (⋆) with a signal drop on the ADC, in keeping with acute pontine infarction. 3D TOF MR angiography (C, D) show complete loss of flow-related signal of the basilar artery (red arrow) indicating occlusion.
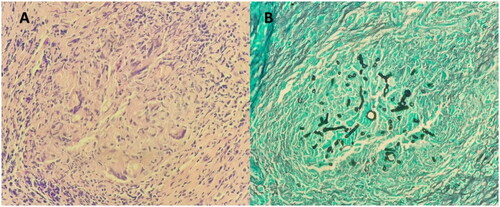
The patient underwent urgent complete bilateral endoscopic sinus surgery. Intraoperatively, all sinuses were filled with fungal debris (mud) and mucin, with extensive bone remodeling and expansion, especially in the sphenoid sinus, without tissue necrosis. (Figure ) Tissue specimens (mucosa and bone) were obtained intraoperatively to confirm the diagnosis. Postoperatively, the patient was admitted to the intensive care unit, intubated, and connected to mechanical ventilation. Preoperative total IgE level in this patient was significantly high (6350 IU/ml). Histopathology report revealed eosinophilic inflammatory cells with mucin and Charcot Leyden crystals and fungal hyphae without vascular and body invasion in addition to the unusual finding of necrotic cellular debris mixed with giant cells (granuloma). (Figure ) The tissue culture yields Aspergillus species. These findings confirmed the diagnosis of AFRS.
Figure 3. Intraoperative endoscopic picture showing the severely expanded sphenoid sinus with indentations of the structures surrounding it. ON: Optic nerve; CC: Cavernous carotid; SF: Sellar floor; ISS: Inter-Sinus septum; CR: Clival recess; PCC: Para clival carotid; * Opico-Carotid recess.
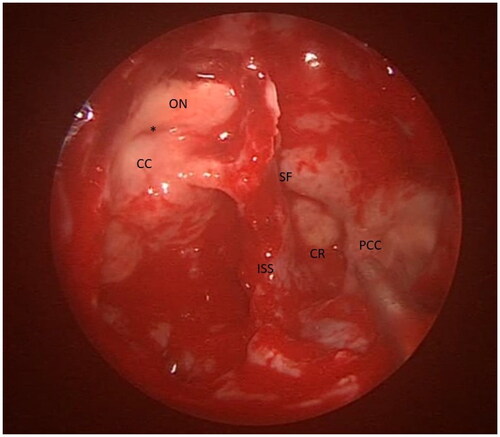
Figure 4. (A) Microscopic examination showed mucosal fragments stained with hematoxylin and eosin stain lined by respiratory mucosa. They are slightly polypoid in shape and show eosinophils surrounded by areas of lightly staining mucin sprinkled with Charcot-Leyden crystals (B) Tissue stained with grocott-–gomori methenamine silver highlights fungal elements. There are fungal elements seen within the granuloma. The fungus has slender septated hyphae, branching at an acute angle. No definite angioinvasion was observed.
Considering the significant morbidity of the patient and the histopathological findings of having granuloma, which is a sign of invasion, the patient was administered amphotericin B concomitantly with a local intranasal steroid to control his allergic fungal sinusitis condition for approximately one month postoperatively. Furthermore, he received a thrombolytic agent to manage basilar artery thrombosis.
After one month, the patient developed intraventricular and mild subarachnoid hemorrhage accompanied by acute hydrocephalus (Figure ) which was notable, and he passed away.
Figure 5. (A, B) Unenhanced axial CT brain images revealed hyperdense material layering within the atria of the lateral ventricle and outlining the sylvain fissures bilaterally indicating intraventricular and subarachnoid hemorrhage with subsequent acute hydrocephalus and transependymal CSF permeation.
Discussion
Patients with AFRS may be comparable to those with chronic sinusitis. The most common symptoms observed upon presentation are nasal obstruction, rhinorrhea, postnasal discharge, and/or headache. A cohort study was conducted to identify the possible complications at the time of presentation. Bozeman et al. classified patients into subgroups based on their demographics, symptoms, and complications. Most of the patients were diagnosed with allergic rhinitis, asthma, and nasal polyposis before the manifestation of allergic fungal complications. The most frequently reported symptoms coexisting with the presenting complications were headache and worsening of previous nasal symptoms [Citation5]. Orbital involvement is a rare and serious complication of AFRS, occurring in 18.3% to 34% of cases, and it can range in severity from proptosis and diplopia to vision impairment [Citation6,Citation7]. Within the existing literature, two mechanisms have been proposed as causes for the impact of AFRS on vision. These mechanisms include expansion and remodeling of the bone structures surrounding the sinuses, which can lead to direct compression of the optic nerve or optic chiasma, as well as irritation and inflammation of the optic nerve caused by a hyperimmune response triggered by fungal antigens [Citation8].
The most common cause of basilar artery infarction is atherosclerosis, which leads to thrombosis or embolic thrombus from cardiac or large arterial sources. The patient had no other risk factors for vascular infarction or thromboembolism. He was cleared from the cardiology department as having a cardiac source of an embolic thrombus. Radiological and intraoperative findings revealed severe compression of the basilar artery.
This may explain the basilar artery infarction in our patient, who was diagnosed with AFRS, which unfortunately resulted in his death. To the best of our knowledge, this is the first reported case of AFRS complicated by basilar artery infarction. The very few reported cases are complications of acute Invasive Fungal Rhinosinusitis (AIFRS) [Citation9].
Therefore, an early diagnosis of AFRS is important to avoid complications. Alarming signs, such as proptosis, in unoperated patients with chronic rhinosinusitis should be considered. Whether a high level of IgE can predict complications in these patients is not clear [Citation5]. The preoperative total IgE level in this patient was very high, which is a major criterion for the diagnosis of AFRS. Alqahtani et al. anticipated that intrapolypoidal white particles (IWP) could provide a basis for future endoscopic diagnostic criteria for the early detection and diagnosis of AFRS in an outpatient setting. Based on this study, the presence of IWP on endoscopic examination predicts AFRS with high sensitivity and moderate specificity. The findings of their study were encouraging since they showed that IWP could be an appealing sign as a reliable endoscopic indicator for the identification of AFRS in outpatient settings [Citation10].
Regarding the radiological findings, a retrospective study including 46 patients were diagnosed with allergic fungal sinusitis. The patients underwent a CT scan of the paranasal sinuses, and the reported findings are as follows: increased intrasinus attenuation, complete opacification, sinus expansion, remodeling, wall thinning, and involvement of adjacent soft tissue, including the medial orbital wall [Citation11]. The radiological features of extensive AFRS can mimic those of AIRFS. The massive expansion and remodeling of bones gives the impression of bone invasion; however, the double-density sign is more obvious in AFRS than in AIFRS. Additionally, intraoperatively these patients showed no intracranial or intraorbital extension with intact borders. Conversely, AIFRS showed very clear bony destruction and erosion. Although mucosal thickening may have been insignificant, signs of invasion on CT were obvious [Citation12].
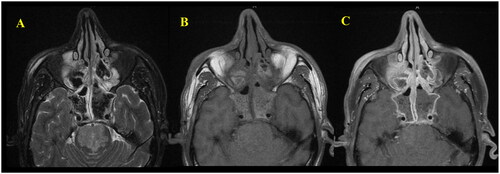
Magnetic Resonance Imaging features include the presence of hypointense/hypoattenuated/low central on the T1 signal. However, signals on T1 might vary depending on water and protein concentration. The central T2 signal void and peripheral T1/T2 enhancement (the enhancement is based on T1 and not T2) are highly specific for AFRS [Citation13]. Similar radiological features were observed in our case, in which imaging showed aggressive infiltrative expansile markedly hypointense T2 signals within the paranasal sinuses, primarily affecting the sphenoid and ethmoid sinuses, with peripheral mucosal enhancement (Figure ). Additionally, acute pontine infarction owing to basilar artery occlusion was detected (Figure ).
Figure 6. (A) Axial T2 FS weighted image shows markedly hypointense T2 signal of the intrasinus content primarily affecting the sphenoid and ethmoid sinuses owing to dense fungal concentration and heavy metals mimic air. (B, C) Axial T1 pre- and postcontrast images revealed peripheral enhancement of the inflamed mucosa within the affected sinuses.
The first histopathological description for AFRS was in 1983 by Katzenstein et al. and they described the histopathological finding of 113 cases of chronic rhinosinusitis and found ‘allergic mucin’—degenerating eosinophils, desquamated respiratory epithelial cells, and Charcot–Leyden crystals in 6% of them [Citation1]. This allergic mucin has the appearance of ‘peanut butter’ at surgery. Hematoxylin and eosin staining of the allergic mucin showed that it comprised masses of many pyknotic eosinophils (eosinophil concretions) surrounded by thin, pink-stained mucus. In addition, when allergic mucin was visualized using Gomori methenamine silver, Charcot–Leyden crystals were identified, as well as spare numbers of fungal hyphae [Citation14]. Gupta et al. based on histopathology, found that some patients with AFRS had chronic invasive granulomatous changes. This indicated invasion; however, no bone or vascular invasion was observed. These patients are named (allergic fungal sinusitis with granuloma). They have a more extensive disease with radiological evidence of extrasinus involvement, higher complication rates, and a higher incidence of recurrence. These patients may require antifungal treatment in addition to local steroids for postoperative management [Citation15]. The histological findings in the present case were the same as the presence of fungal granulomas containing giant cells in the mucosa with fungal elements within the granulomas. This may explain the aggressive nature of the presentation in this patient.
There remains some controversy regarding the treatment of AFRS. However, the general concept of treatment is based on the importance of preoperative and postoperative long-term management of AFRS. Endoscopic sinus procedure is considered the mainstay of management, along with long-term maintenance therapy. Postoperative irrigation of the operated sinuses with local steroids is one of the primary methods for cleaning and effectively controlling the fungal spores that the patient inhales during the postoperative period, as well as controlling mucus buildup within the sinus cavities [Citation16]. Adding antifungal therapy to cases of allergic fungal sinusitis with granuloma requires additional supportive evidence.
Conclusion
Allergic fungal rhinosinusitis is a chronic disorder with significant morbidity and a high recurrence rate that requires long-term follow-up. Although there are no universally accepted treatment protocols for AFRS, preoperative diagnosis and management are crucial. Surgery is considered the mainstay treatment for this condition, along with long-term maintenance therapy, which includes frequent sinus irrigation with local steroids to prevent mucus impaction. A special type of allergic fungal sinusitis with granuloma showed a more aggressive presentation and needs to be treated cautiously by adding antifungal treatments.
Informed consent
The authors confirm that written informed consent has been obtained from the involved patient’s guardian since the condition of the patient was not suitable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Katzenstein ALA, Sale SR, Greenberger PA. Allergic Aspergillus sinusitis: a newly recognized form of sinusitis. J Allergy Clin Immunol. 1983;72(1):89–93. doi: 10.1016/0091-6749(83)90057-X.
- Allphin AL, Strauss M, Abdul‐Karim FW. Allergic fungal sinusitis: problems in diagnosis and treatment. Laryngoscope. 1991;101(8):815–820. doi: 10.1288/00005537-199108000-00003.
- Montone KT. Pathology of fungal rhinosinusitis: a review. Head Neck Pathol. 2016;10(1):40–46. doi: 10.1007/s12105-016-0690-0.
- Bent JP, III, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngology—Head and Neck Surgery. 1994;111(5):580–588.
- Bozeman S, deShazo R, Stringer S, et al. Complications of allergic fungal sinusitis. Am J Med. 2011;124(4):359–368. doi: 10.1016/j.amjmed.2010.12.005.
- Marple BF, Gibbs SR, Newcomer MT, et al. Allergic fungal sinusitis-induced visual loss. Am J Rhinol. 1999;13(3):191–195. doi: 10.2500/105065899781389740.
- Alaraj AM, Al-Faky YH, Alsuhaibani AH. Ophthalmic manifestations of allergic fungal sinusitis. Ophthalmic Plastic Reconstruct Surg. 2018;34(5):463–466. doi: 10.1097/IOP.0000000000001051.
- Alhussien A, Alghulikah A, Albaharna H, et al. Loss of vision outcome for allergic fungal sinusitis: case report and literature review. Ther Adv Allergy Rhinol. 2023;14:27534030231176774. doi: 10.1177/27534030231176774.
- Fu KA, Nguyen PL, Sanossian N. Basilar artery territory stroke secondary to invasive fungal sphenoid sinusitis: a case report and review of the literature. Case Rep Neurol. 2015;7(1):51–58. doi: 10.1159/000380761.
- Al-Qahtani K, Altamimi FN, Al-Harbi MH, et al. The evaluation of the sensitivity and specificity of a new endoscopic diagnostic sign of allergic fungal rhinosinusitis: intrapolypoidal white particles. J Maxillofac Oral Surg. 2021;20(4):612–618. doi: 10.1007/s12663-020-01357-4.
- Salamah MA, Alsarraj M, Alsolami N, et al. Clinical, radiological, and histopathological patterns of allergic fungal sinusitis: a single-center retrospective study. Cureus. 2020;12(7):e9233. doi: 10.7759/cureus.9233.
- Khullar T, Kumar J, Sindhu D, et al. CT imaging features in acute invasive fungal rhinosinusitis-recalling the oblivion in the COVID era. Curr Probl Diagn Radiol. 2022;51(5):798–805. doi: 10.1067/j.cpradiol.2022.02.001.
- Berlucchi M, Pedruzzi B. Allergic fungal sinusitis in children. J Aller Ther. 2012;S5:004. doi: 10.4172/2155-6121.S5-004.
- Schubert MS. Medical treatment of allergic fungal sinusitis. Annals Allergy, Asthma Immunol. 2000;85(2):90–98. doi: 10.1016/S1081-1206(10)62445-3.
- Gupta R, Gupta AK, Patro SK, et al. Allergic fungal rhino sinusitis with granulomas: a new entity? Med Mycol. 2015;53(6):569–575. doi: 10.1093/mmy/myv033.
- Medikeri G, Javer A. Optimal management of allergic fungal rhinosinusitis. J Asthma Allergy. 2020;13:323–332. doi: 10.2147/JAA.S217658.
