Abstract
Bone conduction systems are sometimes not well tolerated due to the tension on the skull. Therefore, a brand non-surgical device was developed, the ADHEAR (MED-EL, Innsbruck, Austria). It is based on a novel fixation system using an adhesive adapter, which allows for a more comfortable and efficient mechanical transmission of the acoustic signal and that can be used as treatment for conductive hearing loss. The purpose of the study is to evaluate the performance of ADHEAR in two patients with unilateral conductive hearing loss. An 8-years-old patient with external ear canal stenosis obtained a functional gain of 28.75 dB, while a 16-years-old patient with Goldenhar syndrome showed a recovery of 53.75 dB. Therefore, both cases suggest that ADHEAR appears to be an effective treatment for pediatric patients who due to the medical condition cannot be treated surgically or fitted with conventional hearing aids.
Introduction
Hearing loss is a worldwide problem, with a huge impact on people ́s health and quality of life. In pediatric patients, even mild to moderate hearing loss, may disrupts speech and language acquisition, school performance and social integration, presenting itself as a barrier to development and education [Citation1]. Specifically, conductive hearing loss, either congenital or acquired, is most often the result from external and middle ear abnormalities, or due to inflammatory or adhesive process [Citation2]. Therefore, the importance of early diagnosis and appropriate intervention is highlighted, whether pharmacological, surgical or through hearing rehabilitation. Regarding hearing rehabilitation, different therapeutic options are available, and among them are bone conduction devices.
Regarding these bone conduction devices, several models have been developed in recent decades, some used as purely external systems that do not require surgery, while others are surgically implanted systems [Citation3], that have a direct fixation to the temporal bone. However, the possible loss of skin integrity, associated risk of infection and insufficient thickness of the bone make these unrecommended for treating some situations, especially in children under five years, and in individuals with surgical contraindications [Citation4,Citation5]. In addition, in some active bone conduction implants, for example Bonebridge (Medel, Austria), sound signal is transmitted by an implantable device through electromagnetic stimuli and vibrations, which requires specific temporal bone anatomy and integrity of skull [Citation5].
Concerning non-surgical devices, these devices can be held into place on the mastoid bone with soft bands or fixed on steel-spring headband or glasses. While their benefits are indisputable, these systems involve high vibration of the skin and bone to ensure the transfer of the acoustic energy to the cochlea, leading to discomfort and complaints of head pressure. Furthermore, the dissipation of energy related to alterations of skin and soft tissues propagation, limits sound transduction to the cochlea. As a result, higher force must be applied by the device to achieve a critical level of amplification [Citation6]. What is more critical is the constant movement of these devices during activities of daily life or physical exercise, which can also contribute to the loss of the sound transmission, as well as poor sound quality [Citation7–9].
To overcome the drawbacks of conventional non-implantable bone conduction devices, ADHEAR (MED-EL, Innsbruck, Austria) has been developed. This bone conduction hearing aid consists of an audio processor that is attached through an adhesive adapter positioned over the mastoid, which can optimize the functional gain and minimize the discomfort caused by the overhead pressure, as well as reduce cosmetic concerns [Citation6,Citation7,Citation10]. In fact, the more discreet and appealing design also helps to improve acceptance by the child and the parents, with impact in the wearing time [Citation2].
Compared to other non-surgical bone conduction hearing aids, the novel fixation system induces a stable placement of the ADHEAR, which improves the transmission of sound to the inner ear [Citation7]. Additionally, the fixation achieved with the adhesive adapter can improve the hearing stimulation. The adhesive adapter was designed to be a non-allergic single use adhesive, positioned over the mastoid, in a hairless area (3, 4). The audio-processor can be easily connected and disconnected from the adapter without having to remove it. The processor has dual microphones and is responsible for the advanced signal processing, and additionally the adhesive bone conduction design, enhance sound resonance in high frequencies, which contribute for improved sound source localization and sound discrimination in unfavorable environments [Citation2,Citation11,Citation12].
However, there are specific criteria for ADHEAR use: i) unilateral or bilateral conductive hearing loss, with bone conduction thresholds up to 25 dB; ii) single-sided deafness.
In these case reports, we evaluate the functional gain (FG) of ADHEAR in two pediatric patients with congenital unilateral conductive hearing loss due to external and middle ear malformations.
Case reports
This study was approved by the Ethics Committee of Faculty of Medicine of University of Coimbra (approval CE-019/2023). As both patients are minors, parents gave written consent to participate, and all procedures were carried out in accordance with the Declaration of Helsinki.
Pure-tone Audiometry (PTA) was performed in a soundproof cabin, and the FG was carried out approximately four weeks after ADHEAR fitting, through loudspeakers positioned 1 m in front of the patients. The sound field measures used were warble tones of frequencies of 0.25 to 2 kHz for case 1 and 0.5 to 4 kHz for case 2. Age-adjusted free-field audiometry was performed in case 1, due to the child’s restricted audibility of higher frequencies. This restriction occurs because current hearing aids are unable to provide substantial gains in these frequencies, and the patient may still have difficulty identifying and differentiating sounds. For case report 2, the patient is 16 years old, and we consider that at this age he can reliably perform free-field audiometry for higher frequencies.
Patients and parents received instructions regarding the use, care, and handling of the device. The use of this bone conduction system was monitored through the processing algorithm that is built into the ADHEAR memory, allowing to control patient´s wearing time.
ADHEAR was used according to the manufacturer’s recommendations (default program), and the volume adjusted to a comfortable level to the patient. Regular appointments were scheduled once a week to monitor the patients progress in adapting to their device.
Case report 1
An 8-year-old female patient with congenital right ear canal stenosis and pre-auricular fistula on the same side, was followed up in a specialized pediatric center of a tertiary hospital. The patient failed the Universal Neonatal Hearing Screening at birth, presenting absent Otoacoustic Emissions and failed the Automated Auditory Evoked Potentials in the right ear. Thus, Auditory Evoked Potentials were performed, showing absence of wave V at intensities below 60 dB in the right ear, while in the left ear, wave V was recorded at 30 dB.
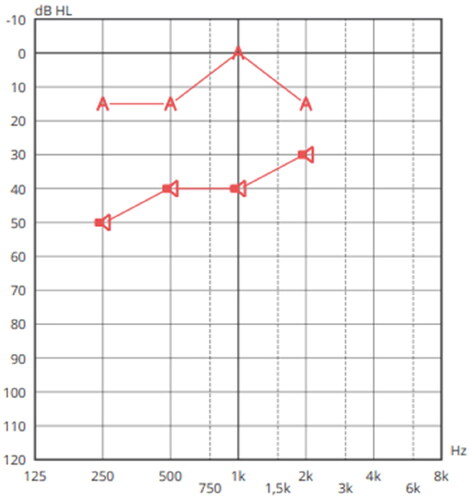
On 30/06/2021, she was referred for hearing rehabilitation. The new audiological evaluation performed included otoscopy, tympanometry, and PTA, confirming a pure tone average in the right ear of 38.75 dB (Figure ). The patient has an average bone conduction hearing thresholds of 4 dB, confirming the candidacy for ADHEAR in the right ear.
Figure 1. Pure-tone audiogram. Mild conductive hearing loss in the right ear and normal hearing in the left ear.
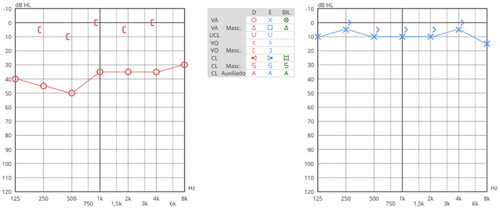
After 4 weeks of usage, free field audiometry with adhesive bone conduction device, were assessed in aided and unaided conditions. The findings showed a mean for the aided condition of 11.25 dB HL, higher than for unaided (40 dB HL) (Figure ), revealing a FG of 28.75 dB. The average wearing time to change the adhesive adapter was 4 to 5 days, with no difficulties reported by the patient parents to replace the adhesive adapter. Furthermore, no adverse skin reactions to the adhesive adapter were observed, as well as no complaints of head pressure or other symptoms. Currently, the child is independent in management of the device.
Case report 2
A 16-year-old male patient with Goldenhar Syndrome had medical follow-up due to several maxillofacial malformations, namely ear canal agenesis, associated with a left unilateral conductive hearing loss, micrognathia, left anophthalmia and hemifacial microsomia.
On 07/06/2021 patient was referred for hearing rehabilitation, and assessment of pure tone thresholds confirmed a normal hearing in right ear (11.25 dB HL) and a moderate type II conductive hearing loss (according to BIAP 02/1) on the left ear (70 dB HL) (Figure ). It was verified that the average bone conduction hearing thresholds in the left ear was 19 dB, in compliance with ADHEAR candidacy. After 4 weeks of ADHEAR use, free field audiometry was performed and revealed a mean FG between aided and unaided conditions of 53.75 dB (Figure ).
Figure 3. Pure-tone Audiometry. Normal hearing in right ear and moderate type II conductive hearing loss in the left ear.
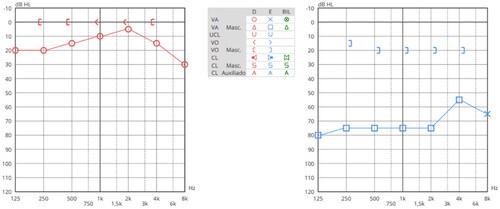
Figure 4. Pure tone audiometry in free field with masking of the right ear. The symbol 
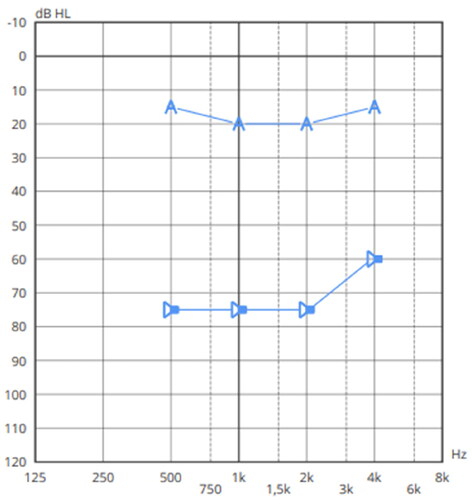
Similar to case report 1, satisfactory FG of ADHEAR was observed, as well as no associated skin complications. Average wearing time to change the adhesive adapter was 6 days and the patient is independent in use, replacement, and management of both components of the device.
Discussion
Non- surgically bone conduction devices have some drawbacks, such as higher aesthetic discomfort, however the main issue is associated to the high pressure applied to the skin and skull of the patients, that is necessary to assure the connection with the transducer [Citation7]. Disadvantages include loss of sound transmission due to unreliable placement and fixation of the transducer, which is determined by the hair between the contact plate and the skin.
The ADHEAR device was developed to overcome these previous disadvantages of non-implantable bone conduction hearing aids. This system benefits include a more reliable and proper placement of the transducer, achieved without pressure. In addition, the distinctly design of the ADHEAR ensures less visibility of the device, improving patient comfort and wellbeing, while maintaining a satisfactory FG. This performance is optimized due to the use of minimal mass of the skin-contact plate obtained with the adhesive adapter, as well as by improving the transmission of vibrations to the inner ear, with no requirement of head pressure.
Management of conductive hearing loss depends on the cause and the degree of the loss, however in children with congenital hearing loss, surgical correction or rehabilitation is still a very challenging procedure. Specifically, in these pediatric cases, ADHEAR has been a promising option, namely for case 1, which has an external ear canal stenosis without any other inflammatory process of middle ear. In case 2, due to the severity of maxillofacial malformations, a bone conduction non-surgical option seems to be also the most appropriate option of hearing rehabilitation to the patient. Our study shows that, in both case reports, a significant improvement in FG with ADHEAR was obtained. Specifically, in case report 1, the FG varied between 15 and 40 dB at frequencies between 0.25 and 2 kHz, with an average improvement of 28.75 dB, while in case report 2, the FG varied between 45 and 60 dB at frequencies between 0.5 and 4 kHz, with an average improvement of 53.75 dB. These results reveal that the use of ADHEAR in these patients resulted in an FG that was relatively higher than that found in a previous study, in which the FG reported was 24.6 dB [Citation13]. Moreover, a study reported that 7 of 9 children who used bone conduction devices on soft band prior to receiving ADHEAR did not accept the conventional system due to pressure on the skull, inconvenience, or stigmatization, while in 8 of these 9 children ADHEAR was successful accepted and was the method chosen to continue the rehabilitation process [Citation2].
In our case reports, we also observed clinically relevant FG in free field audiometry with the ADHEAR at all frequencies (i.e. 0.25 to 2 kHz in case 1, and 0.5 to 4 kHz to case 2) after a short time of acclimatization (4 weeks). Similar results were obtained by Neumann et al. (2019), however in this study 8 of the participants has previous use of other bone conduction devices, namely softbands, which could explain the significant improvements with the device at 1 and 8 kHz in the day of fitting [Citation2]. Based on our results we confirmed that the improvements achieved with ADHEAR exceeded functional hearing gain scores at the first fitting. These findings are particularly important for pediatric hearing rehabilitation, considering that even unilateral hearing loss may impair a child’s language development and verbal communication skills, and if we provide early hearing rehabilitation with optimized FG without any need of time for acclimatization, improved speech and language development can be obtained.
But unlike in our study, Muzzi et al. (2021), only observed a better FG in the low and mid frequencies in adults, when compared to 4 kHz. Considering that ADHEAR´s design was optimized to provide a resonance boost in the high frequencies, this means that ADHEAR performance is probably affected by subjects’ particularities, such as age and hearing loss etiology [Citation8].
Regarding the durability of the adhesive, in both cases it was found to be within the expected range. However, it is still important to highlight the expected inter-individual variability related to variations in the shape of the mastoid, skin type, sweating or a combination of all these factors [Citation11].
Conclusion
In this study, it was observed that ADHEAR may be an alternative and effective treatment for patients with conductive hearing loss, who are not eligible for surgically implanted bone conduction devices and demonstrated that this bone conduction hearing aid system allows a satisfactory audiological performance without requiring pressure on the skin. Based on this, the ADHEAR seems to be an appropriate alternative to conventional bone conduction hearing aids, however, studies with larger samples are needed to validate if acclimatization is effectively immediate and if FG is maintained for a longer follow-up period.
Informed consent statement
Parents or legal representatives of the minor participants gave written consent prior to their participation in the study, and the procedures were carried out in accordance with the Declaration of Helsinki.
Acknowledgments
The authors would like to gratefully acknowledge participants and their parents for generously sharing their time in this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Moeller MP, Tomblin JB. An Introduction to the Outcomes of Children with Hearing Loss Study. Ear Hear. 2015;36(Suppl 1):4S–13S. doi:10.1097/AUD.0000000000000210.
- Neumann K, Thomas JP, Voelter C, et al. A new adhesive bone conduction hearing system effectively treats conductive hearing loss in children. Int J Pediatr Otorhinolaryngol. 2019;122:117–125. Jul doi:10.1016/j.ijporl.2019.03.014.
- Ellsperman SE, Nairn EM, Stucken EZ. Review of bone conduction hearing devices. Audiol Res. 2021;11(2):207–219. doi:10.3390/audiolres11020019.
- Roman S, Nicollas R, Triglia JM. Practice guidelines for bone-anchored hearing aids in children. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128(5):253–258. doi:10.1016/j.anorl.2011.04.005.
- Reinfeldt S, Hakansson B, Taghavi H, et al. New developments in bone-conduction hearing implantes: a review. Med Devices. 2015;8:79–93.
- Almuhawas F, Alzhrani F, Saleh S, et al. Auditory Performance and subjective satisfaction with the ADHEAR system. Audiol Neurootol. 2021;26(1):1–10. doi:10.1159/000507775.
- Favoreel A, Heuninck E, Mansbach A. Audiological benefit and subjective satisfaction of children with the ADHEAR audio processor and adhesive adapter. Int J Pediatr Otorhinolaryngol. 2020;129:109729. doi:10.1016/j.ijporl.2019.109729.
- Muzzi E, Gambacorta V, Lapenna R, et al. Audiological performance of ADHEAR systems in simulated conductive hearing loss: a case series with a review of the existing literature. Audiol Res. 2021;11(4):537–546. doi:10.3390/audiolres11040048.
- Weiss R, Loth A, Leinung M, et al. A new adhesive bone conduction hearing system as a treatment option for transient hearing loss after middle ear surgery. Eur Arch Otorhinolaryngol. 2020;277(3):751–759. doi:10.1007/s00405-019-05769-y.
- Westerkull P. An adhesive bone conduction system, ADHEAR, a new treatment option for conductive hearing losses. J Hear Sci. 2018;8(2):35–43. doi:10.17430/1003045.
- Hirth D, Weiss R, Stöver T, et al. Audiological benefit and subjective satisfaction with the ADHEAR hearing system in children with unilateral conductive hearing loss. Eur Arch Otorhinolaryngol. 2021;278(8):2781–2788. Aug doi:10.1007/s00405-020-06364-2.
- Skarzynski PH, Ratuszniak A, Osinska K, et al. A Comparative study of a novel adhesive bone conduction device and conventional treatment options for conductive hearing loss. Otol Neurotol. 2019;40(7):858–864. Aug doi:10.1097/MAO.0000000000002323.
- Gawliczek T, Munzinger F, Anschuetz L, et al. Unilateral and bilateral audiological benefit with an adhesively attached, noninvasive bone conduction hearing system. Otol Neurotol. 2018;39(8):1025–1030. doi:10.1097/MAO.0000000000001924.