ABSTRACT
The costs of responding and mitigating the COVID-19 pandemic is a critical example of the need for continual investment for global health security (GHS) preparedness in today’s inter-connected world as exemplified earlier with Ebola, Zika, and H1N1. Microbial diversity including endemic and emerging infectious diseases unique to Latin America are well known. When combined with geopolitical, socioeconomic, and environmental factors, especially climate change and human migration, which are expanding the range of disease vectors and pathogens, the risk for infectious disease outbreaks greatly increases. Enhancing GHS requires a greater awareness and cooperation within the region as well as more effective infectious disease surveillance systems. Frameworks such as the International Health Regulations and Global Health Security Agenda underpin policies to strengthen health systems. Greater international cooperation aimed to effectively enhance infectious disease surveillance are pivotal to increasing trust among partner countries and strengthen health security systems and best practices to respond and mitigate infectious disease outbreaks. Here we discuss infectious disease threats and risks associated with the current socioeconomic and political climate that influence GHS in order to demonstrate the need for further investment.
Introduction
The rapid spread of COVID-19 throughout Latin America highlights, yet again, the importance of enhancing global health security engagement in the Latin American region. The combination of inconsistent health care infrastructure, fragile economies, and complex political and economic landscapes has enabled the intense spread of COVID-19 to even the most remote areas of Latin America including indigenous populations along the Amazon that can only be reached by boat (NYT article, Sutton T May 2020). While the coronavirus pandemic exacts a dramatic and deadly toll on the Latin American region today, there have been several epidemics caused by emerging infectious diseases in recent years that have emphasised the need for improvements and continued investment in global health security preparedness in Latin America.
The impact of infectious disease epidemics in the Latin American region has multisectoral and multinational implications including economic crises, political instability, food insecurities, increased migration, and interruptions in other public services like education, transport and public safety (Malamud & Nunez, Citation2020). Global health security threats and associated risks have been assessed in single countries and areas such as Pakistan and the Amazonian forest in Brazil; however, this activity across regions and globally is more difficult (Ellwanger et al., Citation2020; Khalil et al., Citation2017; National Academies of Sciences, Engineering and Medicine, Citation2020). A comparison of what worked and what did not during the initial COVID-19 pandemic in Latin America included eight countries (Garcia et al., Citation2020). These outcomes, including those from climate impact, in turn can have serious economic, public health, and national security consequences for both countries within and outside the region. The COVID-19 pandemic has demonstrated the challenges that all countries face in responding to and mitigating an epidemic or pandemic.
Latin America is commonly defined as the 33 countries and dependencies that cover parts of North America (Mexico), Central and South America, and the Caribbean (7,775,854 square miles (20,139,378 square kilometres). The United Nations estimates the 2020 population of Latin America is over 650 million. Latin America is comprised of low-, middle-, and high-income countries and has the ‘world’s highest income disparities’ which results in major public health challenges; approximately 35% of the region’s population is impoverished and more than 125 million individuals have no access to health care services (Bliss, Citation2009). Here we discuss the political, economic, and environmental factors that contribute to the need for strengthening infectious disease surveillance in Latin America. This will highlight the importance of investing in global health security preparedness in the region.
Emerging and endemic infectious diseases
Latin America is especially vulnerable to suffer from endemic infectious diseases and those with epidemic and pandemic potential due to healthcare infrastructure that has limited capacity and is highly variable within the region (Bliss, Citation2009; Jones et al., Citation2008). While some individuals living in urban settings can better afford quality healthcare services, the lower-income populations and those living in remote areas often have very limited access to healthcare. This high variability in the healthcare infrastructure translates to limited surveillance capacity particularly among the most vulnerable populations who are often at the greatest risk of both endemic and emerging infectious diseases. The surveillance capacity relies on robust capability for laboratory diagnostic testing including specific molecular and immunological assays. Otherwise diagnoses are typically based on clinical signs and symptoms testing in the absence of laboratory diagnostics.
The Latin American region has extensive microbial diversity and is endemic for a wide array of infectious agents including dengue, chikungunya, malaria and tuberculosis. While these endemic diseases exact an ongoing burden on the healthcare infrastructure, they also periodically cause major epidemics that spread rapidly throughout the region, resulting in significant morbidity and mortality and further taxing already overburdened health care systems. The region is at particularly high risk of emerging and re-emerging infectious diseases as evidenced by the increased number of disease events of potential international public health concern. In 2014, the WHO cited 93 public health events of potential international concern in the Latin American region where over half (47 events) were caused specifically by chikungunya and other zoonotic pathogens causing geographically widespread impact affecting 27 countries and territories (Espinal et al., Citation2016). More importantly, the 2014–2016 outbreak of Ebola virus in West Africa primed health security preparedness and response in Latin America, which the Pan American Health Organization (PAHO) implemented an approach aligned with the International Health Regulations (IHR) framework that created a regional task force with coordinated emergency operations and stockpile centres, and elevated this plan to heads of state (Espinal et al., Citation2016). COVID-19 also provided another opportunity for PAHO to reinforce public health core capacities and transparency for reporting and notifying diseases through regional coordination.
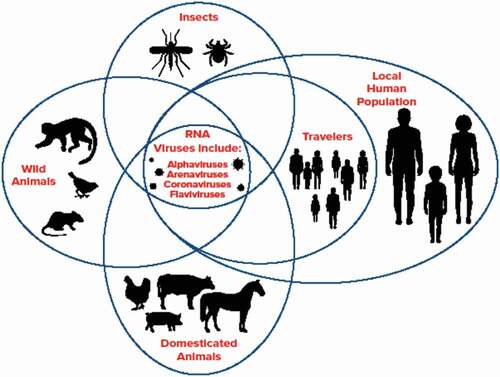
Arena, alpha, and flaviviruses are RNA viruses many of which are endemic in South America, are diverse in nature, and can adapt easily to new hosts creating zoonotic threats (Carrasco-Hernandez et al., Citation2017). Since more than 70% of emerging diseases are caused by zoonotic agents, it is of great importance to enhance the capacity for detection and diagnosis in the areas that they are most likely to emerge. One group of arenaviruses (South American Haemorrhagic Fever viruses: Chapare, Guanarito, Junin, Machupo, and Sabia), which are on the U.S. Federal Select Agent list due to their potential threat is also endemic in South America (MacDermott & Ksiazek, Citation2020). The rodent reservoir diversity in South America suggests the ease of spread of these arenaviruses when they become infected and shed virus in their faeces, urine, and saliva (Han et al., Citation2015). Examples of RNA viruses in South America are present among animal, human, and insect populations ().
Figure 1. Example RNA viruses are at the focal point of animal, human, and vector interactions in South America. Illustration used with permission from Penn State University
In addition to these arenaviruses, endemic flaviviruses such as dengue virus (DENV), yellow fever virus (YFV), and Zika virus (ZIKV), and alphaviruses such as chikungunya (CHIKV), Venezuelan Equine Encephalitis (VEE), Eastern Equine Encephalitis (EEE), and Mayaro are major public health challenges in Latin America that can easily spread to North America and establish autochthonous transmission due to the presence of competent mosquito vectors. These agents also have the potential to threaten the safe blood supply through transfusion-transmitted disease (Levi, Citation2018). In addition, tick-borne disease has the potential to spread rickettsia and other agents. These pathogens have the potential to incur global biological threats, particularly in light of the limited capacity of health care infrastructure in the Latin America region if gone undetected and without timely response capabilities can incur global biological threats.
Of particular concern in Latin America are vector-borne diseases of mosquitoes, ticks, other arthropods, and snails. Vector-borne diseases are considered, in general, to be sensitive to climate and are increasing globally (Jones et al., Citation2008). The top vector-borne diseases in Latin America for numbers of people affected are concerned are malaria, leishmaniasis, dengue fever, chikungunya virus, Chagas disease and schistosomiasis (Caminade et al., Citation2019). Dengue epidemics have recurred in 3- to 5-year cycles in the region of the Americas. Dengue cases have increased since 2000; in 2017 alone, over 483,000 cases and 253 deaths were reported. As of 2018, there are an estimated 145 million people in 21 countries of the Americas living in areas at risk for malaria (PAHO, Citation2018). In 2017 alone, some 680,000 cases were reported in the region. The numbers of Zika cases between 2015 and January 2018 were 583,451 (suspected) and 223,477 (confirmed) and 3,720 confirmed cases of congenital Zika syndrome. Nearly 6 million people in Latin America suffer chronic impacts of Chagas disease that is especially prevalent among families whose dwellings are unprotected and in poor condition, and within indigenous communities. Essentially all vector-borne diseases are reported to be on the rise in Latin America by the Pan American Health Organization (PAHO, Citation2018).
The 2015 Zika epidemic exemplifies the global vulnerability to diseases and potential for diseases to rapidly spread from Latin America and the Caribbean. The first cases of Zika were reported in Brazil in March 2015 and within 18 months it had spread throughout South and Central America, the Caribbean, Florida and Texas in the US (Likos et al., Citation2016). In July 2016, the first cases of Zika in the continental US were reported in the Wynwood neighbourhood of Miami-Dade County, Florida, and by 22 July 2016, the Florida Department of Health had identified 321 cases (Likos et al., Citation2016). While the first cases of Zika were reported in July 2016, subsequent mathematical modelling estimated that the Zika virus was actually introduced into the Wynwood neighbourhood between 3 and 5 months before it was detected (Marini et al., Citation2017). Similarly, genomic epidemiologic research also demonstrated that ‘at least four introductions, but potentially as many as 40, contributed to the outbreak in Florida, and that local transmission is likely to have started in the spring of 2016’ (Grubaugh et al., Citation2017). The Zika outbreak demonstrates that an infectious agent can rapidly spread to North America (United States and territories) after introduction or emergence in Latin America and Caribbean, and also to establish transmission within North America several months prior to actual detection.
COVID-19 demonstrates a scenario in which a highly transmissible respiratory pathogen rapidly spreads person-to-person throughout Latin America, resulting in significant public health and socioeconomic impacts throughout the region. This coronavirus, which is also an RNA virus called the 2019-novel coronavirus (2019-nCoV) and now designated as the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in four cases associated with a wholesale market in Wuhan, China on 29 December 2019 (Li, Citation2020). The disease caused by SARS-CoV-2 is now called coronavirus disease 2019 (COVID-19) and zoonotic. Latin America’s first case was confirmed in Brazil in late February 2020 through a traveller returning to Sao Paulo, a major air hub, from Italy and it is expected the health care systems and economy will be impacted further than in wealthier countries (Rodriguez-Morales et al., Citation2020). The current COVID-19 pandemic, which is expected to peak in Latin America in late 2020, demonstrated the speed an infectious disease can spread globally through air travel and transmission by pre-symptomatic and asymptomatic individuals. Though the SARS-CoV-2 did not emerge in Latin America, the limited capacity of the healthcare infrastructure for preparedness, diagnosis and response has led to rapid spread throughout the region despite early awareness of the impact that the COVID-19 pandemic was having in other parts of the world. Furthermore, both COVID-19 and Zika now circulate among asymptomatic individuals which reinforces the importance for laboratory diagnostics and active surveillance.
While outbreaks that cause human disease are a constant reminder that infectious diseases do not respect borders, other viruses can be transferred between animals and humans (zoonotic) and a variety of animal and plant pathogens. The close proximity and trade of agricultural products especially livestock movement can facilitate a pathogen such as foot-and-mouth disease virus (FMDV) to easily cross borders.
FMDV has not occurred in Central America, and it was eliminated in North and South America. The Pan-American Highway which is continuous from Alaska, US to Argentina provides an ideal trade route and access points that would in turn boost economies. In 1973, the highway was completed except for a last stretch of area along the border of Panama and Colombia. This area is known as the Darien Gap and dense jungle provides a natural geographic land barrier. One risk for completing the highway was the potential for FMDV to cross from Colombia to Panama (Blackwell, Citation1980). Today, the Darien Gap is preserved largely as national park land and has land routes used for foot traffic by migrants.
A recent investigation of the origins of the 2009 H1N1 pandemic influenza virus found extensive diversity in the influenza viruses circulating in Mexican swine with several viruses containing genetic segments from viruses that originated in Eurasia. These sequences indicated that viruses with genetic composition similar to the 2009 H1N1 pandemic virus had been circulating in the swine population in Mexico for over 10 years. These investigators also found that the movement of viruses from Eurasia into Mexico ‘closely follows the direction of the global trade of live swine’ (Mena et al., Citation2016).
Wheat blast, which is a fungal disease caused by Magnaporthe oryzae, originated in Brazil and was found in Bangladesh in 2016 (Islam et al., Citation2016). This fungus has the potential to devastate grains, a major global staple and central to food security in the US and the world. In 2016, the New World Screwworm, which is another agricultural pest, was speculated to have entered the US from Latin America and nearly devastated the endangered Key Deer population in the Florida Keys (Delgado et al., Citation2016). Further, new diseases are being discovered in the region but are largely unreported due to lack of surveillance capabilities (Blohm, Citation2018).
Ecological state and environmental change
When considering some parameters to assess disease emergence such as population growth, land-use change, agriculture, and the increase in the wildlife-human interface, Latin America is rapidly approaching the levels of all of the drivers seen that increase infectious disease emergence in other parts of the world. In particular, Amazonian forests have a substantial influence on regional and global climates (Malhi et al., Citation2008). Deforestation can itself be a driver of climate change and a positive feedback on externally forced climate change, which then can impact other regions of the world leading to environmental factors that increase the threat of infectious diseases. In particular, arthropod-borne diseases will continue to move into more temperate regions (Bartlow et al., Citation2019). With parallel repercussion, as deforestation in the Amazonian Basin continues to be converted to agricultural land, especially for crop, livestock, and urban development, the use of ‘bushmeat,’ which is the consumption of wild animals for food, is rising (Karesh & Noble, Citation2009; Nasi et al., Citation2008). Serological evidence in bats also suggests that certain filoviruses and henipaviruses may circulate in the region (Schulz et al., Citation2020). Consumption and trade of wildlife is also exemplified in the current COVID-19 outbreak where bats and pangolins may be intermediate reservoirs between the SARS-COV-2 virus and humans (Andersen et al., Citation2020; Zhang et al., Citation2020). While in some areas of South American bushmeat has been considered economically feasible for feeding human populations, it may not be sustainable (Nielsen et al., Citation2018). When the supply and demand of bushmeat consumption results in the loss of natural habitat, the chances of a new infectious disease emerging increase dramatically. Depletion of wildlife has negative repercussions not only for rural villages that depend on this source for nutrition, but it also impacts the habitat long term as species often perform important and irreplaceable ecosystem functions (Kurten, Citation2013). Evidence indicates that biodiversity loss frequently increases disease transmission. Areas of depleting naturally high biodiversity such as the Amazon Basin may serve as a source pool for new pathogens (Keesing et al., Citation2010; Myers et al., Citation2000). Current evidence indicates that preserving intact ecosystems and their endemic biodiversity should generally reduce the prevalence of infectious disease (Keesing et al., Citation2010). Zoonotic spillover requires several factors to align. This includes the ecological, epidemiological and behavioural determinants for pathogen exposure, and human factors that affect susceptibility to infection (Plowright et al., Citation2017). Recent socio-economic and political trends in Latin America have led to rapid environmental changes. Not only do these environmental changes such as deforestation have the capacity to negatively impact climate, they can greatly increase the risk for emerging infectious diseases.
Political, economic, and social factors
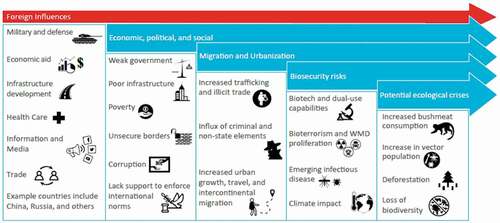
Latin America has a complex history of political and socioeconomic issues. Together, when taken into account with the emerging infectious disease threat and social factors such as human migration, global travel, population growth, and ever-changing land use, the setting for a major outbreak and subsequent crisis is presented ().
Figure 2. Potential global health security threats and risks in Latin America. Various factors directly influence the biological and health threats that impact the population and environmental dynamics
Political and security concerns took a pivotal turn during the Cold War through the Cuban and Soviet forged political alliances. Today, ties to Russia, along with China, and Iran remain strong amongst socialist leaders throughout Latin America (Ellis, Citation2018; Harris, Citation2018). The economic makeup of Latin America varies widely throughout the region and is influenced by the oil, mining, and other domestic and foreign industries. The majority of Latin America and Caribbean countries are in the high- and middle-high-income categories as defined by the World Bank.
Despite this, the region continues to suffer from unstable socio-political and economic environments that allow inroads for foreign influences. Through heavy investments in infrastructure in Latin America, China has the ability to exert significant economic influence through capital investments, credit supply, and purchasing of large amounts of raw materials (Chaguaceda, Citation2019). Similarly, Russia is also boosting its influence in Latin America utilising various strategies, particularly through technical-military collaboration and bilateral geopolitical alliances. Weapons sales and training by Russia are increasing, which is exemplified by the recent purchase of Russian air equipment by Argentina, Colombia, and Mexico, which they have historically purchased from the US (Chaguaceda, Citation2019). Relatedly, the biotechnology market in Latin America follows the rest of North America, Europe, and the Asian Pacific and has a demonstrated record of international collaboration (León-de La et al., Citation2018). While there is considerable advanced scientific capacity in many Latin American countries, funding is scarce, which allows opportunities for foreign influences to fulfil these gaps.
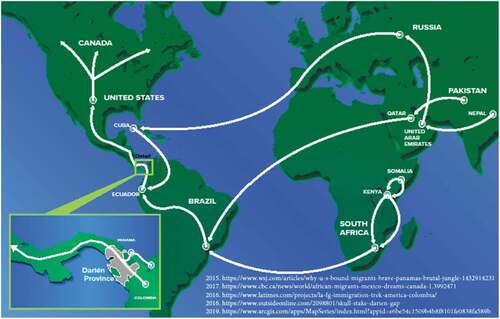
The foreign influences that form these economic and political alliances do not provide a social foundation to increase the general well-being of its citizens. As a result, people emigrate seeking better opportunities. Today, open-source data shows that human migration into the continental U.S. and Canada often occurs through Panama via the Darien Gap from points worldwide including Moscow, Russia; Sao Paulo, Brazil; and Johannesburg, South Africa ().
Conclusion
Peace and conflict are often at the hands of security, economy, health, and diplomacy. Biosecurity and infectious disease surveillance are key areas for investment as they are directly linked to health and economic security, which in turn leads to greater stability. The COVID-19 pandemic serves as a token reminder that the cost to prepare is less than the cost to respond.
Cooperative engagement between nations has a proven track record for strengthening surveillance capabilities to detect, diagnose and report infectious zoonotic diseases (National Academies of Sciences, Engineering and Medicine, Citation2020). Both country-level infrastructure development and regional cooperation need to be organised to better prioritise these issues which global partnerships can positively contribute to both facets. Infectious disease surveillance and reporting systems exist as evidenced by PAHO but detection and diagnostic capabilities and capacities are still needed at the operational level. In order to enhance capabilities and capacities to prevent, detect, and respond to biosecurity threats overall, more engagements need to be formalised. This begins with the agencies and countries that fund biosecurity, threat reduction, and cooperative engagement programmes, as well as public–private partnerships in the health, biotechnology, and life science industries to tailor surveillance, reporting, and response capacities. At the top level for awareness and national interests, country leaders need to prioritise building cooperation across respective regional interests. Private sectors and academic universities should be engaged to continue building health security capabilities and capacities. Addressing global health security risks and threats requires continual trust building to attain sustainable financial investment.
Acknowledgement
This article was written without any funding. The authors thank the reviewers and editors for their critique and insights. Special appreciation to Carl Newman and John Jacocks for their guidance and Garrett Dalton for his graphic artwork.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Kenneth B. Yeh
Kenneth B. Yeh is a senior director at MRIGlobal, which is an independent nonprofit research organization. He has created partnerships and reinforced best practices to develop technical solutions in the areas of biodefense, biotechnology, and global health security. During 2009-2014, Kenny developed and implemented over 12 bioresearch studies in Kazakhstan under the Biological Threat Reduction Program which fostered subsequent publications on cooperative research. His published work includes related panel discussions on first movers in biotechnology, sharing of data and sample material, and research in high-containment facilities. This work complements his earlier industry experience that was key to developing and deploying a U.S. DoD real-time qPCR platform for identifying biological agents in the field. His real-world experience includes: onsite technical support to clients during the 2001 anthrax attacks, AMI building site decontamination, and UNMOVIC trained biological WMD expert. He has BS (University of Maryland) and MS (Clemson) degrees in microbiology.
Falgunee K. Parekh
Dr. Parekh is Founder and Principal Scientist of EpiPointe, a woman and minority-owned small business that develops epidemiologic solutions to combat global infectious diseases. She is an infectious disease epidemiologist with more than 20 years of experience in the development and implementation of biosurveillance, field epidemiology and clinical studies in international settings including South America, South Asia, West and East Africa, and Central Asia. Dr. Parekh’s research experience is focused on assessing the epidemiology, disease risk factors, and epidemic potential of endemic and emerging infectious diseases including malaria, Lassa Fever, brucellosis, dengue, Zika, and tuberculosis. She has served as Principal Investigator or Subject Matter Expert for several cooperative research and threat reduction programs in Africa and Central Asia. Dr. Parekh also works on development and implementation of clinical trials for vaccines and interventions targeting infectious diseases including malaria, H1N1, and Zika. Dr. Parekh also currently serves on Leadership Committee as the Treasurer of the Global Health Security Agenda Consortium.
Brooke Borgert
Brooke Borgert is currently a medical student at the Edward Via College of Osteopathic Medicine (VCOM). She completed her Master of Science at Georgetown University in Biohazardous Threat Agents and Emerging Infectious Diseases. She subsequently worked as the Lab Manager in the Infectious Disease Dynamics lab at the University of Florida. She has 10 years of experience researching infectious disease dynamics with a focus on respiratory pathogen transmission dynamics and vector competence and spatiotemporal dynamics related to vector-borne diseases. Brooke has also applied her experience to strengthen health and security engagement in Latin America.
Gene G. Olinger
Gene G. Olinger is a principal science advisor at MRIGlobal in the Life Sciences Division. He also serves as an adjunct associate professor at Boston University School of Medicine and is a full professor at the Kansas City University College of Biosciences. He is a seasoned scientist with 20+ years of experience leading complex in vitro and in vivo studies in BSL-3/BSL-4 laboratories to drive delivery of vaccines, clinical diagnostics, and countermeasure developments to emerging pathogens. As a subject matter expert in immunology, pathogenesis, host-pathogen interactions, virology, and vaccinology, he has been engaged in developing partnerships to better the health globally. He is a thought leader in medical countermeasure development, biosurveillance, and biocontainment, evidenced by multiple patents, 100+ peer-reviewed published manuscripts, abstracts, and reviews. For the past five years, Dr. Olinger has been focused on One Health program development with partners in the U.S., Africa, India, South and Central America. Beyond his current efforts to improve workforce training in diagnostics, he is initiating a strategic program on the future Bioeconomy.
Jeanne M. Fair
Jeanne Fair, Ph.D. is a scientist at Los Alamos National Laboratory with a research focus in epidemiology and animal disease ecology. In 2009, Dr. Fair was a lead analyst for the Department of Homeland Security’s modeling of the H1N1 influenza pandemic. From 2013-2016 she was on assignment as a Science Program Manager with the Defense Threat Reduction Agency’s Biological Threat Reduction Program working with Central Asia and the Middle East. Dr. Fair is dedicated to cooperative biological engagement for strengthening capabilities for biosurveillance around the world through scientific collaborations and research partnerships. She has over 100 scientific publications in the fields of ornithology, epidemiology, and science collaboration.
References
- Andersen, K.G., Rambaut, A., Lipkin, W.I., Holmes, E.C., & Garry, R.F. (2020). The proximal origin of SARS-CoV-2. Nature Medicine, 26 (4), 450–452. Accessed April 1, 2020. https://doi.org/https://doi.org/10.1038/s41591-020-0820-9.
- Bartlow, W.A., Manore, C., Xu, C., Kaufeld, A.K., Del Valle, S., Ziemann, A., Fairchild, G., & Fair, J.M. (2019). Forecasting zoonotic infectious disease response to climate change: Mosquito vectors and a changing environment. Veterinary Sciences, 6(2), 40. https://doi.org/https://doi.org/10.3390/vetsci6020040
- Blackwell, J.H. (1980). Internationalism and survival of foot-and-mouth disease virus in cattle and food products. Journal of Dairy Science, 63(6), 1019–1030. https://doi.org/https://doi.org/10.3168/jds.S0022-0302(80)83040-2
- Bliss, K.E., 2009. Health in Latin America and the Caribbean: Challenges and opportunities for US engagement: A report by the CSIS Global Health Policy Center. Center for Strategic and International Studies.
- Blohm, G.M. The challenge of conducting arbovirus surveillance in Venezuela. SACEMA Quarterly. (2018, June). Retrieved September 10, 2018, from http://sacemaquarterly.com/wp-content/uploads/2018/06/Gabriela_The-challenge-of-conducting_article-4.pdf
- Caminade, C., McIntyre, K.M., & Jones, A.E. (2019, January). Impact of recent and future climate change on vector‐borne diseases. Annals of the New York Academy of Sciences, 1436(1), 157. https://doi.org/https://doi.org/10.1111/nyas.13950
- Carrasco-Hernandez, R., Jácome, R., López Vidal, Y., & Ponce De León, S. (2017). Are RNA viruses candidate agents for the next global pandemic? A review. ILAR Journal. December. 15. 58(3), 343–358. (Accessed April 1, 2020.). https://doi.org/https://doi.org/10.1093/ilar/ilx026
- Chaguaceda, A. The bear comes to the West: The Russian agenda in Latin America. [online] Global Americans. 2019. Available at: https://theglobalamericans.org/2019/03/the-bear-comes-to-the-west-the-russian-agenda-in-latin-america/[Accessed 3 Nov. 2019].
- Delgado, A., Hennessey, M., & His, D. (2016) . Investigation into introduction of New World Screwworm into Florida Keys. USDA. https://www.aphis.usda.gov/stakeholders/downloads/2017/nws-epi-report.pdf. Accessed April 23, 2020
- Ellis, E. (2018). The impact of China on the Latin American security environment. Revista Da Escola De Guerra Naval, 24(2), 456–462. (Accessed October 31, 2020) https://revista.egn.mar.mil.br/index.php/revistadaegn/issue/view/74
- Ellwanger, J.H., Kulmann-Leal, B., Kaminski, V.L., Valverde-Villegas, J.M., Veiga, A.B.G., Spilki, F.R., … Almeida, S.E. (2020). Beyond diversity loss and climate change: Impacts of Amazon deforestation on infectious diseases and public health. Anais Da Academia Brasileira De Ciências, 92, 1. https://doi.org/https://doi.org/10.1590/0001-3765202020191375
- Espinal, M., Aldighieri, S., John, R.S., Becerra-Posada, F., & Etienne, C. (2016). International health regulations, Ebola, and emerging infectious diseases in Latin America and the Caribbean. American Journal of Public Health, 106(2), 279–282. https://doi.org/https://doi.org/10.2105/AJPH.2015.302969
- Garcia, P.J., Alarcón, A., Bayer, A., Buss, P.M., Guerra, G., Ribeiro, H., Rojas, K., Saenz, R., Snyder, N.S.D., Solimano, G., & Torres, R., 2020. Perspective Piece COVID-19 Response in Latin America. The American Society of Tropical Medicine and Hygiene.
- Grubaugh, N.D., Ladner, J.T., Kraemer, M.U.G., Dudas, G., Tan, A.L., Gangavarapu, K., Wiley, M.R., White, S., Thézé, J., Magnani, D.M., Prieto, K., Reyes, D., Bingham, A.M., Paul, L.M., Robles-Sikisaka, R., Oliveira, G., Pronty, D., Barcellona, C.M., Metsky, H.C., Baniecki, M.L., & Andersen, K.G. (2017). Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature Retrieved October 19, 2018; from. 546(7658), 401–405. https://doi.org/https://doi.org/10.1038/nature22400
- Han, B.A., Schmidt, J.P., Bowden, S.E., & Drake, J.M. (2015). Rodent reservoirs of future zoonotic diseases. Proceedings of the National Academy of Sciences, 112(22), 7039–7044. https://doi.org/https://doi.org/10.1073/pnas.1501598112
- Harris, K. (2018, August). America’s Newest Southern Neighbor? An Analysis of Russian Influence in Latin America (Rep.). Retrieved September 28, 2018, from American Security Project website: https://www.americansecurityproject.org/wp-content/uploads/2018/08/Ref-0214-Americas-Newest-Southern-Neighbor.pdf
- Islam, M.T., Croll, D., Gladieux, P., Soanes, D.M., Persoons, A., Bhattacharjee, P., Hossain, M.S., Gupta, D.R., Rahman, M.M., Mahboob, M.G., Cook, N. (2016). Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biology, 14(1), 1–11. https://doi.org/https://doi.org/10.1186/s12915-016-0309-7
- Jones, K.E., Patel, N.G., Levy, M.A., Storeygard, A., Balk, D., Gittleman, J.L., & Daszak, P. (2008). Global trends in emerging infectious diseases. Nature, 451(7181), 990–993. Accessed January 31, 2020. https://doi.org/https://doi.org/10.1038/nature06536
- Karesh, W.B., & Noble, E. (2009). The bushmeat trade: Increased opportunities for transmission of zoonotic disease. Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine:, 76(5), 429–434. Accessed September 26, 2019. https://doi.org/https://doi.org/10.1002/msj.20139
- Keesing, F., Belden, L.K., Daszak, P., Dobson, A., Harvell, C.D., Holt, R.D., Hudson, P., Jolles, A., Jones, K.E., Mitchell, C.E., Myers, S.S., Bogich, T., & Ostfeld, R.S. (2010). Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature, 468(7324), 647. https://doi.org/https://doi.org/10.1038/nature09575
- Khalil, A.T., Ali, M., Tanveer, F., Ovais, M., Idrees, M., Shinwari, Z.K., & Hollenbeck, J.E. (2017). Emerging viral infections in Pakistan: Issues, concerns, and future prospects. Health Security, 15(3), 268–281. https://doi.org/https://doi.org/10.1089/hs.2016.0072
- Kurten, E.L. (2013). Cascading effects of contemporaneous defaunation on tropical forest communities. Biological Conservation, 163, 22–32. https://doi.org/https://doi.org/10.1016/j.biocon.2013.04.025
- León-de La, O.D.I., Thorsteinsdóttir, H., Calderón-Salinas, J., & Hermes-Lima, M. (2018). The rise of health biotechnology research in Latin America: A scientometric analysis of health biotechnology production and impact in Argentina, Brazil, Chile, Colombia, Cuba and Mexico. Plos One, 13(2), e0191267. Accessed April 23, 2020. https://doi.org/https://doi.org/10.1371/journal.pone.0191267
- Levi, J.E. (2018). Emerging infectious agents and blood safety in Latin America. Frontiers in Medicine, 5(14), 71. https://doi.org/https://doi.org/10.3389/fmed.2018.00071
- Li, Q. (2020). (2020). An outbreak of NCIP (2019-nCoV) infection in China—wuhan, Hubei province, 2019−. China CDC Weekly, 2(5), 79–80. https://doi.org/https://doi.org/10.46234/ccdcw2020.022
- Likos, A., Griffin, I., Bingham, A.M., Stanek, D., Fischer, M., White, S., Hamilton, J., Eisenstein, L., Atrubin, D., Mulay, P., Scott, B., Jenkins, P., Fernandez, D., Rico, E., Gillis, L., Jean, R., Cone, M., Blackmore, C., McAllister, J., Vasquez, C., & Philip, C. (2016). Local Mosquito-Borne Transmission of Zika Virus — Miami-Dade and Broward Counties, Florida, June–August 2016. MMWR. Morbidity and Mortality Weekly Report, 65(38), 1032–1038. https://doi.org/http://dx.doi.org/10.15585/mmwr.mm6538e1
- MacDermott, N., & Ksiazek, T.G. (2020). South American haemorrhagic fevers. BMJ Best Practices. Retrieved 20 April 2021 from https://bestpractice.bmj.com/topics/en-gb/1612
- Malamud, C., & Nunez, R. 2020. COVID-19 in Latin America: Political challenges, trials for health systems and economic uncertainty. Elcano Royal Institute. http://www.realinstitutoelcano.org/wps/wcm/connect/d9568b35-adcb-4a5b-9dca-b706f1e0a65d/ARI36-2020-Malamud-Nunez-COVID-19-Latin+America-political-challenges-trials-for-health-systems-and-economic-uncertainty.pdf?MOD=AJPERES&CACHEID=d9568b35-adcb-4a5b-9dca-b706f1e0a65d. Accessed 23 OCT 2020.
- Malhi, Y., Roberts, J.T., Betts, R.A., Killeen, T.J., Li, W., & Nobre, C.A. (2008). Climate Change, Deforestation, and the Fate of the Amazon. SCIENCE, 319(5860), 169. https://doi.org/https://doi.org/10.1126/science.1146961
- Marini, G., Guzzetta, G., Rosà, R., & Merler, S. (2017, September 14). First outbreak of Zika virus in the continental United States: A modelling analysis. Eurosurveillance, 22(37), 30612. PubMed PMID: 28933344; PubMed Central PMCID: PMC5607655. https://doi.org/https://doi.org/10.2807/1560-7917.ES.2017.22.37.30612
- Mena, I., Nelson, M.I., Quezada-Monroy, F., Dutta, J., Cortes-Fernández, R., Lara-Puente, J.H., Castro-Peralta, F., Cunha, L.F., Trovão, N.S., Lozano-Dubernard, B., Rambaut, A., Van Bakel, H., & García-Sastre, A. (2016, June 28). Origins of the 2009 H1N1 influenza pandemic in swine in Mexico. Elife, 5, e16777. https://doi.org/https://doi.org/10.7554/eLife.16777
- Myers, N., Mittermeier, R.A., Mittermeier, C.G., Da Fonseca, G.A.B., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403(6772), 853–858. https://doi.org/https://doi.org/10.1038/35002501
- Nasi, R., Brown, D., Wilkie, D., Bennett, E., Tutin, C., Van Tol, G., & Christophersen, T., Conservation and use of wildlife-based resources: The bushmeat crisis. In: Technical Series no. 33. 2008. Secretariat of the Convention on Biological Diversity & Center for International Forestry Research (CIFOR), Montreal; Bogor, pp. 50. https://www.cbd.int/doc/publications/cbd-ts-33-en.pdf Accessed September 26, 2019.
- National Academies of Sciences, Engineering, and Medicine. 2020. A strategic vision for biological threat reduction: The U.S. Department of Defense and Beyond. Washington DC: The National Academies Press. https://doi.org/https://doi.org/10.17226/25681.
- Nielsen, M.R., Meilby, H., Smith-Hall, C., Pouliot, M., & Treue, T. (2018). The importance of wild meat in the global south. Ecological Economics, 146(1), 696–705. https://doi.org/https://doi.org/10.1016/j.ecolecon.2017.12.018
- PAHO 2018. Plan of action on entomology and vector control 2018-2023. 70th Session of the Regional Committee of WHO for the Americas. Pan American Health Organization. https://www.paho.org/hq/index.php?option=com_docman&view=download&category_slug=56-directing-council-english-9964&alias=45774-cd56-11-e-poa-entomology-774&Itemid=270&lang=en
- Plowright, R.K., Parrish, C.R., McCallum, H., Hudson, P.J., Ko, A.I., Graham, A.L., & Lloyd-Smith, J.O. (2017). Pathways to zoonotic spillover. Nature Reviews. Microbiology, 15(8), 502. https://doi.org/https://doi.org/10.1038/nrmicro.2017.45
- Rodriguez-Morales, A.J., Gallego, V., Escalera-Antezana, J.P., Mendez, C.A., Zambrano, L.I., Franco-Paredes, C., Suárez, J.A., Rodriguez-Enciso, H.D., Balbin-Ramon, G.J., Savio-Larriera, E., Risquez, A. COVID-19 in Latin America: The implications of the first confirmed case in Brazil. Travel Medicine and Infectious Disease. (2020).February. 29. 101613. (Accessed March 31, 2020.). 35. https://doi.org/https://doi.org/10.1016/j.tmaid.2020.101613
- Schulz, J.E., Seifert, S.N., Thompson, J.T., Avanzato, V., Sterling, S.L., Yan, L., Letko, M.C., Matson, M.J., Fischer, R.J., Tremeau-Bravard, A., Seetahal, J.F., Ramkissoon, V., Foster, J., Goldstein, T., Anthony, S.J., Epstein, J.H., Laing, E.D., Broder, C.C., Carrington, C.V.F., Schountz, T., & Munster, V.J. (2020). February. 8. Serological Evidence for Henipa-like and Filo-like Viruses in Trinidad Bats. The Journal of Infectious Diseases, (Accessed April 1, 2020). 221(Suppl._4). S375–S382. https://doi.org/https://doi.org/10.1093/infdis/jiz648
- Zhang, T., Wu, Q., & Zhang, Z. (2020). Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Current Biology, 30 (7), 1346–1351.e2. Accessed April 1, 2020. https://doi.org/https://doi.org/10.1016/j.cub.2020.03.022.