Abstract
We characterized the complete chloroplast genome sequence of Ligularia fischeri, a collection from Halla Mountain in Jeju Island, Korea. The plants are utilized as edible functional plant species harbouring useful antioxidant compounds in family Asteraceae. De novo assembly with whole genome sequencing data of L. fischeri completed the chloroplast genome of 151 133 bp long, which included two inverted repeats (IRs) blocks of 24 831 bp, separated by the large single-copy block of 83 238 bp and small single-copy block of 18 233 bp. The genome encoded 113 genes consisting of 80 protein-coding genes, 29 tRNA genes and 4 rRNA genes. Phylogenetic analysis with protein coding gene sequences of reported Asteraceae chloroplast genomes revealed a close relationship of L. fischeri with Jacobaea vulgaris, a weed species world-widely distributed.
Introduction
Ligularia fischeri, called Gomchi in Korea, is a perennial herbal plant belonging to family Asteraceae. In Korea, this plant has been used as a wild edible herbal plant and is mainly distributed in damp shady regions of high altitudes more than at least 600 m in the eastern part of Korea (Choi et al. Citation2007; Shang et al. Citation2010; Song et al. Citation2014). In addition, the leaves of this species have been used in Korea for medicinal purposes such as treatment of jaundice, scarlet fever, rheumatoid arthritis and hepatic function failure, owing to their antioxidant compounds, like Korean ginseng (Jeong et al. Citation1998; Lee et al. Citation2002, Citation2015; Park & Choi Citation2007; Bae et al. Citation2009). Nevertheless, large cultivation of L. fischeri in farm field is difficult, due to the limitation of cultivatable regions (Song et al. Citation2014). In this study, we characterized the complete chloroplast genome sequence of L. fischeri to contribute to further physiological, molecular and phylogenetical studies of this plant.
A plant material of L. fischeri, endemic in Jeju Island Korea, was provided by Hanteak Botanical Garden (http://www.hantaek.co.kr/), Yongin, Korea. Genomic DNA was extracted from fresh leaves following a modified cetyltrimethylammonium bromide (CTAB) protocol (Allen et al. Citation2006). The quality and quantity of the DNA were examined using a NonDrop ND-1000 (NanoDrop Technologies, Inc., Wilmington, DE, USA). A pair-end (PE) library was constructed and sequenced using an Illumina MiSeq platform by Lab Genomics, Inc. (Seongnam, Korea). Approximately 1.01 Gb of sequencing data were obtained, and de novo assembled by a CLC genome assembler (v. beta 4.6, CLC Inc., Rarhus, Denmark), as reported in Kim et al. (Citation2015a, Citation2015b). Compared with the chloroplast sequence of Artemisia frigida (NC_020607) as a reference, eight representative chloroplast contigs were selected and joined into a single draft sequence. The draft sequence was validated and corrected by PE read mapping. DOGMA software was used for annotation of protein-coding genes in the chloroplast genome (Wyman et al. Citation2004), and manual confirmation process was carried out by BLAST searches.
The complete chloroplast genome of L. fischeri was circular DNA molecule of 151 133 bp in length, which was separated into a large single copy region of 83 238 bp, a small single copy region of 18 233 bp and a pair of inverted repeats (IRa and IRb) of 24 831 bp. The genome contained a total of 113 genes including 80 protein-coding genes, 29 tRNA genes and 4 rRNA genes, and showed the GC content of 37.5%. Among the genes identified, 7 protein-coding, 7 tRNA and 4 rRNA genes were completely duplicated in the IR regions. The complete chloroplast genome of L. fischeri was submitted into GenBank with accession number KT988070, which is firstly reported chloroplast genome sequence in genus Ligularia.
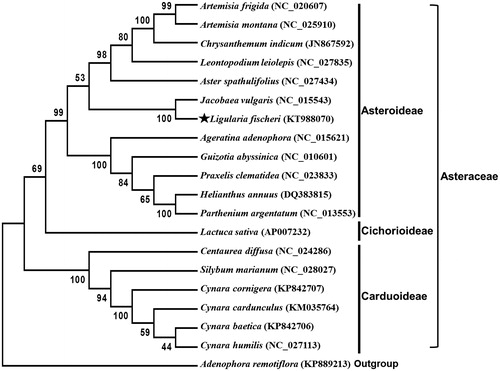
The phylogenetic analysis of L. fischeri was performed by comparison with 45 protein-coding gene sequences derived from chloroplast genome sequences of other 18 species in family Asteraceae. Phylogenetic tree was generated by a maximum likelihood analysis of MEGA 6.0 program (Tamura et al. Citation2013) using 1000 bootstrap replicates and revealed that L. fischeri was placed mostly close to Jacobeae vulgaris (synonym Senecio jacobaea), a biennial or perennial Asteraceae weed widely distributed in Asia, Europe, America, Africa and Australia (McLaren et al. Citation2000; Jacobs & Sing Citation2009) ().
Figure 1. Maximum likelihood phylogenetic tree of L. fischeri with other 19 species based on 45 protein-coding gene sequences. Numbers in the nodes are the bootstrap values from 1000 replicates. The chloroplast sequence of Adenophora remotiflora (KP889213) in family Campanulaceae was set as an outgroup.
Disclosure statement
This research was supported by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (NRF-2015M3A9A5030733).
References
- Allen G, Flores-Vergara M, Krasynanski S, Kumar S, Thompson W. 2006. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat Protoc. 1:2320–2325.
- Bae J, Yu S, Kim Y, Chon S, Kim B, Heo B. 2009. Physiological activity of methanol extracts from Ligularia fischeri and their hyperplasia inhibition activity of cancer cell. J Bio-Environ Control 18:67–73.
- Choi EM, Ding Y, Nguyen HT, Park SH, Kim YH. 2007. Antioxidant activity of Gomchi (Ligularia fischeri) leaves. Food Sci Biotechnol. 16:710–714.
- Jacobs J, Sing S. 2009. Ecology and management of tansy ragwort (Senecio jacobaea L.); [cited 2015 Nov 9). Available from: http://www.treesearch.fs.fed.us/pubs/34173.
- Jeong SW, Kim EJ, Hwangbo HJ, Ham SS. 1998. Effects of Ligularia fischeri extracts on oxidation of low density lipoprotein. Korean J Food Sci Technol. 30:1214–1221.
- Kim K, Lee SC, Lee J, Lee HO, Joh HJ, Kim NH, Park HS, Yang TJ. 2015a. Comprehensive survey of genetic diversity in chloroplast genomes and 45S nrDNAs within Panax ginseng species. PLos One 10:e0117159.
- Kim K, Lee SC, Lee J, Yu Y, Yang K, Choi BS, Koh HJ, Waminal NE, Choi HI, Kim NH, et al. 2015b. Complete chloroplast and ribosomal sequences for 30 accessions elucidate evolution of Oryza AA genome species. Sci Rep. 5:15655.
- Lee KT, Koo SJ, Jung SH, Choi J, Jung HJ, Park HJ. 2002. Structure of three new terpenoids, spiciformisins a and b, and monocyclosqualene, isolated from the herbs of Ligularia fischeri var. spiciformis and cytotoxicity. Arch Pharmacol Res. 25:820–823.
- Lee SM, Bae BS, Park HW, Ahn NG, Cho BG, Cho YL, Kwak YS. 2015. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J Ginseng Res. 39:384–391.
- McLaren DA, Ireson JE, Kwong RM. 2000. Biological control of ragwort (Senecio jacobaea L.) in Australia. In: Spencer NR, editor. Proceedings of the X International Symposium on Biological Control of Weeds (July 4–14, 1999). Montana State University, 67–79; [cited 2015 Nov 9). Available from: http://www.invasive.org/proceedings/pdfs/10_67-79.pdf.
- Park HJ, Choi MY. 2007. Antinocicepetive effects of 3, 4-Dicaffeoyl Quinic acid of Ligularia fischeri var. spiciformis. Korean J Plant Resources 20:221–225.
- Shang YF, Kim SM, Song DG, Pan CH, Lee WJ, Um BH. 2010. Isolation and identification of antioxidant compounds from Ligularia fischeri. J Food Sci 75:C530–C535.
- Song KS, Jeon KS, Kim CH, Yoon JH, Park YB, Kim JJ. 2014. Effect of shading level on growth and morphological characteristics of Ligularia fischeri seedling. Protect Horticulture Plant Factory. 23:88–94.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30:2725–2729.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics 20:3252–3255.
