Abstract
Glyptothorax zainaensis, a small-sized benthic fish which mainly distributes in the Nujiang River, Lancangjiang river and their tributaries in China. In the present study, the complete mitochondrial genome of G. zainaensis was sequenced to be 16 537 bp in length, including 13 protein-coding genes, 2 ribosomal RNAs, 22 transfer RNAs, a control region and the origin of the light strand replication. The overall nucleotide composition was 31.17% A, 25.95% T, 27.48% C and 15.40% G, with an A + T bias of 57.13%. The gene composition and the structural arrangement of the G. zainaensis complete mitochondrial DNA were identical to most of the other vertebrates. This will provide a useful tool for understanding the genetic diversity, population structure and conservation status of G. zainaensis in the future.
Introduction
Mitochondrial DNA (mtDNA) gene order was proposed to be quite conserved within vertebrates based on the gene order of the initial genome sequence (Anderson et al. Citation1981; Bibb et al. Citation1981). Glyptothorax zainaensis (Siluriformes: Sisoridae) is an endemic fish species which mainly distributes in the Nujiang River, Lancangjiang river and their tributaries in China. In recent years, the natural resource of this species has seriously declined, as a result of overharvesting, water contamination and especially dam construction (Jiang et al. Citation2007; Huang et al. Citation2011). In the long run, a good understanding of the genetic diversity and population structure of G. zainaensis is required in order to establish adequate management plans for the conservation of this species. To address these topics, we determined the complete mitochondrial genome sequence of G. zainaensis for the first time.
Specimens of G. zainaensis were collected from Yunnan Province, Nujiang River (25°51′22.81″N. 98°51′0.78″E) in March 2015 and preserved in 95% ethanol until Total genomic DNA was isolated from the caudal fin by proteinase K digestion followed by the standard phenol/chloroform method (Sambrook and Russell, Citation2001) and visualized on 1.5% agarose gel. Twenty sets of primers were designed for PCR amplification on the basis of aligned mitogenome sequences of Glyptothorax trilineatus with (Accession NC_021608.1). In order to avoid errors of assembly, the complete mtDNA genome was aligned and checked with 4 reported mtDNA genome sequences of Sisoridae species Glyptothorax sinensis (Accession NC_024672.1); Glyptothorax fokiensis fokiensis (Accession NC_018769.1); Glyptothorax trilineatus (Accession NC_021608.1). The assembled sequence was analyzed using the software MitoAnnotator (Iwasaki et al. Citation2013) and nucleotide composition was calculated by MEGA6 (Tamura et al. Citation2013).
The complete mtDNA sequence of G. zainaensis reported here has been deposited in GenBank under the accession number KU212205. The mitochondrial genome of G. zainaensis is a circular molecule of 16 537 nucleotides, which is similar to other vertebrates, including 13 protein-coding genes, 2 ribosomal RNA genes, 22 transfer RNA genes and a non-coding control region (D-loop). The overall nucleotide composition is 31.17%, 25.95%, 27.48% and 15.40% for A, T, C and G, with an A + T content of 57.13%, respectively. Except for a single protein-coding gene (ND6) and eight t RNA genes (t RNAGln, t RNAAla, t RNAAsn, t RNACys, t RNATyr, t RNASer (UCN), tRNAGlu and tRNAPro) encoded on the L-strand. All the other genes were encoded on the H-strand. The first non-coding region is 888 bp between tRNAPro and tRNAPhe, and the second one is the origin of light-strand replication, which extends up to 30 bp. It is located in a cluster of five tRNA genes (the WANCY region) between tRNAAsn and tRNACys gene.
Furthermore, the termination codon varies with TAA, TA, T or TAG. Virtually, all of the 13 protein-coding genes show the regular initiation codon ATG with the sole exception of COI which started with GTG. Six protein-coding genes terminated with the complete stop codon TAA (ND1, COI, ATPase8, ND4L and ND5) or TAG (ND6), while the rest ended with incomplete stop codon T (ND2, COII, COIII, ND3, ND4 and Cytb) or TA (ATPase 6)which is quite typical among mtDNA genes in other fishes (Zhou et al. Citation2012; Wang et al. Citation2013).
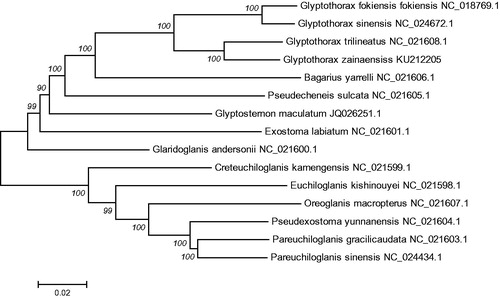
In addition, the mtDNA sequences of 14 species of fishes were downloaded from Gen Bank, Oreoglanis macropterus (Accession NC_021607.1), Pseudexostoma yunnanensis (Accession NC_021604.1), Pareuchiloglanis gracilicaudata (Accession NC_021603.1), Pareuchiloglanis sinensis (Accession NC_024434.1) were used as an out-group for phylogenetic analysis. Phylogenetic analyses were performed using the neighbor joining (NJ) in MEGA 6.0 (Kumar et al. Citation2008). The tree topologies based on complete mt DNA sequences in this study were identical and were statistically supported by high bootstrap and posterior probability values (). The mitogenome data provided strong support that G. zainaensis was clustered together and form a sister group with Glyptothorax trilineatus (Accession NC_021608.1), Glyptothorax sinensis (Accession NC_024672.1), Glyptothorax fokiensis fokiensis (Accession NC_018769.1), Bagarius yarrelli (Accession NC_021606.1), Pseudecheneis sulcata (Accession NC_021605.1), Glyptosternon maculatum (Accession JQ026251.1), Exostoma labiatum (Accession NC_021601.1), Glaridoglanis andersonii (Accession NC_021600.1). The phylogenetic analyses yielded convincing evidence that the G. zainaensis is located at the bottom position in Glyptothorax species, and that the tandem repeats might be a feature of the ancestral teleost lineage.
Figure 1. The consensus phylogenetic relationship of the Glyptothorax zainaensis with other Sisoridae species. Oreoglanis macropterus, Pseudexostoma yunnanensis, Pareuchiloglanis gracilicaudata and Pareuchiloglanis sinensis were used as an out-group. The numbers along the branches are Bayesian posterior probability and bootstrap values for NJ, estimated for concatenated mitochondrial protein sequences.
Declaration of interest
The authors are grateful to thank the Special Project of National Science and Technology Fundamental Work of China (Grant No. 2012FY112700). The authors report no conflicts of interest. The authors alone are responsible for the content and authorship of this report.
References
- Anderson S, Bankier AT, Barrell BG, De Bruijn M, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sange F, et al. 1981. Sequence and organization of the human mitochondrial genome. Nature. 290:457–465
- Bibb MJ, Van Etten RA, Wright CT, Walberg MW, Clayton DA. 1981. Sequence and gene organization of mouse mitochondrial DNA. Cell. 26:167–180.
- Huang F, Xia ZQ, Zhang N, Lu ZH. 2011. Does hydrologic regime affect fish diversity – a case study of the Yangtze Basin (China). Environ Biol Fish. 10:1–16.
- Iwasaki W, Fukunaga T, Isagozawa R, Yamada K, Maeda Y, Satoh TP, Sado T, et al. 2013. MitoFish and MitoAnnotator: a mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol Biol Evol. 30:2531–2540.
- Jiang H, Xie SG, Zhao WQ, Chang JB. 2007. Changes of fish assemblages after construction of Eetan Reservoir in Yalong River (in Chinese). Acta Hydrobiol Sin. 31:532–539.
- Kumar S, Dudley J, Nie M, Tamura K. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinformatics. 9:299–306.
- Sambrook J, Russell D. 2001. Molecular cloning – a laboratory 138 manual. 3rd ed. New York: Cold Spring Harbor Laboratory Press.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30:2725–2729.
- Wang XY, Cao L, Liang HW, Li Z, Zou GW. 2013. Mitochondrial DNA sequence of shorthead catfish (Pelteobagrus eupogon). Mitochondrial DNA. 24:1–2.
- Zhou C, Wang D, Yu M, He, S. 2012. The complete mitochondrial genome of Glyptothorax fukiensis fukiensis (Teleostei, Siluriformes:Sisoridae). Mitochondrial DNA. 24:414–416.
