Abstract
The complete mitochondrial genome of the shoal chub (Macrhybopsis hyostoma) was determined to be 16,899 bp and contained 22 tRNA genes, 2 rRNA genes and 1 control region. The whole genome base composition was 30.5% A, 28.5% T, 24.9% C and 16.1 G. This complete mitochondrial genome provides essential molecular markers for resolving phylogeny and future conservation efforts.
Macrhybopsis chubs are a genus of the subfamily Leuciscinae, which consist of small-bodied fishes that are typically obligate river species (Galat et al. Citation2005). The shoal chub (Macrhybopsis hyostoma) serves as key food chain species for the endangered pallid sturgeon (Scaphirhynchus albus) (Gerrity et al. Citation2006; Herman et al. Citation2008) and has been experiencing significant population declines throughout their ranges, which may be attributable to anthropogenic disturbances (Hesse Citation1994; Steffensen et al. Citation2014). Previous molecular studies were unable to resolve the phylogeny of Macrhybopsis chubs with singular mitochondrial markers, making identifying populations that are susceptible to anthropogenic disturbances difficult (Nagle & Simons Citation2012).
Here, we report the complete mitogenome of the shoal chub, M. hyostoma. The shoal chub was collected from the Loup River near Pawnee Park in Columbus, Nebraska, and is part of the ichthyology collection at the University of Kansas Biodiversity Institute (KUI 41380). This mitogenome will establish a solid basis to resolve phylogenetic confusion within this genus and may aid future conservation measures.
Genomic DNA was extracted and purified from fin tissue using the Qiagen DNeasy Blood and Tissue Kit (Germantown, MD) for Genotyping by Sequencing (GBS). PCR free libraries were constructed with a TruSeq PCR Free library protocol and sequenced on an Illumina NextSeq500 (Kearneysville, WV) at the USGS Leetown Science Facility. Sequences were assembled using Velvet (Zerbino & Birney Citation2008), aligned with Mega 6.06 (Tamura et al. Citation2013) and annotated with MitoFish (Iwasaki et al. Citation2013). DOGMA was used to verify annotation and identify start and stop codons (Wyman et al. Citation2004) ().
Table 1. Characteristics of the mitochondrial genome of the shoal chub (Macrhybopsis hyostoma).
The total length of the mitogenome was 16,899 bp (GenBank Accession No. KX139437). The mitogenomes of these two chubs consisted of 22 tRNA genes, 2 rRNA genes and 1 control region. Fourteen of the tRNA genes were encoded on the heavy (H) strand along with all of the protein-coding genes except NADH dehydrogenase subunit 6. The whole genome base composition was 30.5% A, 28.5% T, 24.9% C and 16.1 G, which is analogous to other teleost mitochondrial genomes which exhibit A/T bias (Wang et al. Citation2013). The putative control region was located between tRNAPro and tRNAPhe and was 1,240 bp long.
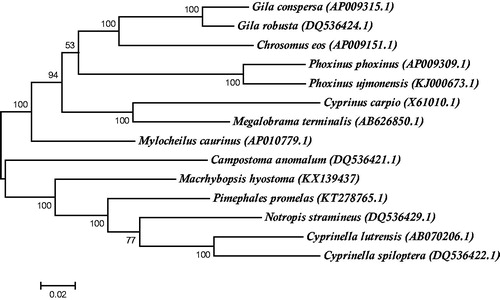
To investigate the position of M. hyostoma within Leuciscinae, a maximum likelihood tree based on 14 complete mitochondrial genomes was constructed using MEGA6 under the GTR + G+I model with 500 bootstrap replicates (Pattengale et al. Citation2010; Tamura et al. Citation2013) (). This maximum likelihood tree phylogenetically positioned M. hyostoma as a sister clade to the Notropin clade supporting previous morphological phylogenetic analysis (Cavender & Coburn Citation1992).
Acknowledgements
We are sincerely grateful to George Cunningham and Ryan Vencil for assistance with specimen collection.
Disclosure Statement
The authors report no conflict of interest. The authors are solely responsible for the content and writing of this manuscript. Use of trade, product, or firm names does not imply endorsement by the U.S. Government.
Additional information
Funding
References
- Cavender TM, Coburn M. 1992. Phylogenetic relationships of North American Cyprinidae. In: Mayden RL, editor. Systematics, historical ecology, and North American freshwater fishes. Standford: Standford University Press; p. 293–327.
- Galat DL, Berry CR, Gardner WM, Hendrickson JC, Mestl GE, Power GJ, Stone C, Winston MR. 2005. Spatiotemporal patterns and changes in Missouri River fishes. Am Fish Soc Symp. 45:249–291.
- Gerrity PC, Guy CS, Gardner WM. 2006. Juvenile pallid sturgeon are piscivorous: a call for conserving native cyprinids. Trans Am Fish Soc. 135:604–609.
- Herman P, Plauck A, Utrup N, Hill T. 2008. Three Year summary age and growth report for sicklefin chub (Macrohybopsis meeki). Pallid Sturgeon Population Assessment Project and Associated Fish Community Monitoring for the Missouri River. Columbia, MO: United States Fish and Wildlife Service Columbia National Fish and Wildlife Conservation Office.
- Hesse L. 1994. The status of Nebraska fishes in the Missouri River, 5. Selected chubs and minnows (Cyprinidae): sicklefin chub (Macrhybopsis meeki), sturgeon chub (M. gelida), silver chub (M. storeriana), speckled chub (M. aestivalis), flathead chub (Platygobio gracilis), plains minnow (Hybognathus placitis), and western silvery minnow (H. argyritis). Trans Nebraska Acad Sci. 21:99–108.
- Iwasaki W, Fukunaga T, Isagozawa R, Yamada K, Maeda Y, Satoh TP, Sado T, Mabuchi K, Takeshima H, Miya M, Nishida M. 2013. MitoFish and MitoAnnotator: a mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol Biol Evol. 30:2531–2540.
- Nagle BC, Simons AM. 2012. Rapid diversification in the North American minnow genus Nocomis. Mol Biol Evol. 63:639–649.
- Pattengale ND, Alipour M, Bininda-Emonds OR, Moret BM, Stamatakis A. 2010. How many bootstrap replicates are necessary? J Comput Biol. 173:337–354.
- Steffensen KD, Shuman DA, Stukel S. 2014. The status of fishes in the Missouri River, Nebraska: shoal chub (Macrhybopsis hyostoma), sturgeon chub (M. gelida), sicklefin chub (M. meeki), silver chub (M. storeriana), flathead chub (Platygobio gracilis), plains minnow (Hybognathus placitus), western silvery minnow (H. argyritis), and brassy minnow (H. hankinsoni). Trans Nebr Acad Sci. 34:49–67.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol. 30:2725–2729.
- Wang B, Ji P, Wang J, Sun J, Wang C, Xu P, Sun X. 2013. The complete mitochondrial genome of the Oujiang color carp, Cyprinus carpio var. color (Cypriniformes, Cyprinidae). Mitochondrial DNA. 24:19–21.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20:3252–3255.
- Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829.