Abstract
We describe six sets of primers for amplifying the mitochondrial control region (CR) of various bird species belonging to nine families of the order Passeriformes. These overlapping primers, with both short and long fragments, yielded an approximately 1 kb fragment of the CR. The short length of the amplified product makes the primers suitable for degraded DNA samples. These primers were used on a wide range of bird species for amplifying and sequencing the highly variable portion of the CR. The primers proved to be a valuable tool for studying the population genetics of bird species. The different sets of primers provide the researcher a choice of markers for different sample types and studies.
Introduction
Molecular genetics is being used increasingly in various conservation applications. It is useful in determining the level of genetic variation, phylogenetics and phylogeography with a high level of accuracy. Due to the presence of conserved sites in mitochondrial DNA (mtDNA) regions such as the 12s, 16s and cytochrome b gene, these fragments are widely used for species identification (Kocher et al. Citation1989; Wan et al. Citation2004). The mtDNA control region is helpful in identifying significant conservation units for those species that are historically isolated (Wenink et al. Citation1994; Gupta et al. Citation2015). Besides, knowledge about the variability of the hypervariable control region is helpful in identifying lower-category taxa such as species or sub-species. The mitochondrial genome is highly variable in avian species (Wenink et al. Citation1994). The control region (CR) often evolves faster than the rest of the mitochondrial genome (Baker & Marshall Citation1997). This variability of the CR has made it a powerful tool for studying the genetic structures of populations. Because of the high variability in the CR and the least conserved sites, designing conserved primers is often a challenging task (Arif & Khan Citation2009). Moreover, bird genetics largely involves non-invasively collected biological samples (shed feathers, faeces, shells of broken/hatched eggs). Amplifying long fragments of the CR from such opportunistically collected samples is a challenging task. A set of primers amplifying shorter fragments will be useful for such samples (Gupta et al. Citation2014). In this work, we describe a panel of primers for amplification of the mtDNA CR of selected bird species from degraded DNA.
Materials and methods
Primer design
The complete mitochondrial genomes of 28 bird species belonging to nine families (Muscapidae, Polioptilidae, Emberizidae, Estrildidae, Viduidae, Nectariniidae, Passeridae, Prunellidae, Fringillidae) were obtained from GenBank and aligned using Clustal W multiple alignments (Thompson et al. Citation1994). On the basis of sequence similarity, we designed four forward and four reverse primers targeting the CR ().
Table 1. Primer sequences for the amplification of control region of bird species and expected length of their amplicons.
Sample collection, DNA extraction and amplification
We collected shed feathers, broken egg shells and blood samples of 28 different bird species through a field survey. Genomic DNA was extracted using the standard phenol–chloroform method (Sambrook et al. Citation1989) and subjected to PCR amplification using the primer combination described in . The amplification was carried out in a 20 μl reaction volume containing 1 μl of the extracted DNA, 100 μM of dNTPs, 4 pmol of each primer, 1.5 mM MgCl2, 0.5 units of AmpliTaq Gold (Life Technologies) and 1 × PCR buffer (10 mM Tris–HCl, pH 8.3, and 50 mM KCl). The PCR conditions were the following: initial denaturation at 95 °C for 10 min, followed by 35 cycles of denaturation at 95 °C for 45 s, annealing at 56 °C for 45 s and extension at 72 °C for 90 s. The final extension was at 72 °C for 10 min. The PCR products were electrophoresed on 2% agarose gel, stained with ethidium bromide (0.5 mg/ml) and visualized under a UV transilluminator. The PCR products obtained were sequenced directly in 3130 Genetic Analyzer (Applied Biosystems) from both directions.
Results and conclusion
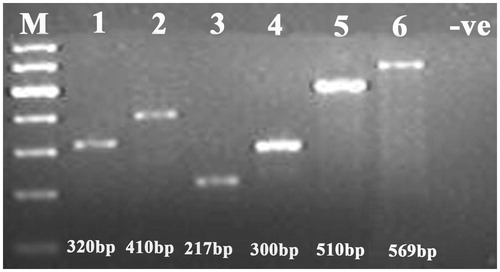
DNA from 28 different bird species was successfully amplified. The primers described in this study were useful in generating the DNA sequence database and were helpful in identifying species and sub-species and in phylogeographic differentiation. The use of different lengths of the CR amplicon in single PCR was a useful approach to amplifying the combination of short and longer DNA fragments found in degraded samples (). The short length of the amplified product makes these primers suitable for highly degraded samples. Therefore, the overlapping fragments generated by the primer set were helpful in covering the longer portions of the CR. The PCR conditions described in this article worked consistently for all the primers mentioned. Besides, these primers could be used with a large range of bird species. Hence, they are can be a valuable tool for studying population genetics and identifying evolutionarily significant units (ESUs).
Acknowledgements
This study was funded by Scientific and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India through grant number SR/SO/AS-85/2012. We thank Dr V. B. Mathur, Director, WII; Dr G. S. Rawat, Dean, WII; Dr Pratap Singh, WII; Dr Y. V. Jhala, WII and Dr Dhananjai Mohan, Additional PCCF, Uttarakhand Forest Department for their kind support and Suresh Kumar Rana for assistance with sample collection. We thank the forest departments of Jammu & Kashmir, Uttarakhand, Sikkim, West Bengal and Arunachal Pradesh for according permissions to conduct the study.
Disclosure statement
There is no conflict of interest. All authors have read and agree with the content of the paper.
Additional information
Funding
References
- Arif IA, Khan HA. 2009. Molecular markers for biodiversity analysis of wildlife animals: a brief review. Anim Biodivers Conserv. 32:9–17.
- Baker AJ, Marshall HD. 1997. Mitochondrial control region sequences as tools for understanding evolution. In: Mindell, DP, editor. Avian molecular evolution and systematics. San Diego: Academic Press; p. 51–82.
- Gupta SK, Kumar A, Gaur A, Hussain SA. 2015. Detection of 40 bp insertion-deletion (INDEL) in mitochondrial control region among sambar (Rusa unicolor) populations in India. BMC Res Notes. 8:581.
- Gupta SK, Kumar A, Hussain SA. 2014. Novel primers for sequencing of the complete mitochondrial cytochrome b gene of ungulates using non-invasive and degraded biological samples. Conserv Genet Resour. 6:499–501.
- Kocher TD, Thomas WK, Meyer A, Edwards SV, Paabo S, Villablanca FX, Wilson AC. 1989. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci USA. 86:6196–6200.
- Sambrook J, Fritschi EF, Maniatis T. 1989. Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press.
- Thompson JD, Higgins DG, Gibson TJ. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680.
- Wan QH, Wu H, Fujihara T, Fang SG. 2004. Which genetic marker for which conservation genetics issue? Electrophoresis. 25:2165–2176.
- Wenink PW, Baker AJ, Tilanus MJ. 1994. Mitochondrial control-region in two shorebird species, the turnstone and the dunlin, and their utility in population genetic studies. Mol Biol Evol. 11:22–31.