Abstract
The live-bearing fish Xenotoca variata is representative of the viviparous Goodeinae subfamily (Goodeidae) from central Mexico. The mitogenome of the X. variata consist of 37 genes in 16,462 bp. Comparing with X. eiseni, the most related of the mitogenomes included, an identity of 91.1% was found and trna-met duplication in X. eiseni is absent in X. variata. The mitogenome provide important information for future studies in evolution of the live-bearing subfamily.
The species Xenotoca variata (Bean Citation1887), is representative of the live-bearing subfamily Goodeinae (Goodeidae) and is distributed in central Mexico: in the Middle Lerma River, Zacapu basin, Cuitzeo Lake, Chapala Lake, Pánuco and Aguanaval basins (Domínguez-Domínguez Citation2008). Goodeines have been a model for the study in evolution, due their peculiar characteristics of breeding strategies and embryo development. Important genetic divergences (Domínguez-Domínguez et al. Citation2010; Corona-Santiago & Domínguez-Domínguez Citation2013), sexual selection (Moyaho et al. Citation2004; Ritchie et al. Citation2007), substantial phenotypic plasticity (Fitzsimons Citation1972; Macías-Garcia Citation1998) have been observed among X. variata populations. Hence, the aim of this work is the characterization of mitochondrial genome of Xenotoca variata, which could provide relevant information for future studies.
For mitogenome sequencing we use a sample tissue (pectoral fin) of Xenotoca variata from Huingo spring (19°54′44.0″N 100°50′00.3″W), Cuitzeo basin, and were storage in the Colección de Peces de la Universidad Michoacana-UMSNH, Mexico (Voucher specimen: CPUM-7031). Briefly DNA was sheared using a Covaris S2 (Woburn, MA) ultrasonicator, and Illumina (Illumina, San Diego, CA) adapters were ligated on using methods derived from Fisher et al. (Citation2011), but using adapters equivalent to Illumina TruSeq with 10nt indexes (Faircloth & Glenn Citation2012). Genomic DNA was subjected to sequencing at the Georgia Genomics Facility (University of Georgia). Reads quality was analyze using FastQC (Andrews Citation2010), adapters and poorly quality sequences were trimmed using Trimmomatic v0.36 (Bolger et al. Citation2014) to assembly using SOAPdenovo2 (Luo et al. Citation2012). Genome annotation was performed using MitoAnnotator (Iwasaki et al. Citation2013) but the position of all tRNA genes was confirmed using tRNAscanSE v1.21 (Schattner et al. Citation2005). Phylogenetic reconstruction was performed under Neighbour-Joining analysis including 14 species of the Cyprinodontiformes order available on GenBank. The analysis was conducted with a full alignment built in MAFFT v7.222 (Katoh et al. Citation2002).
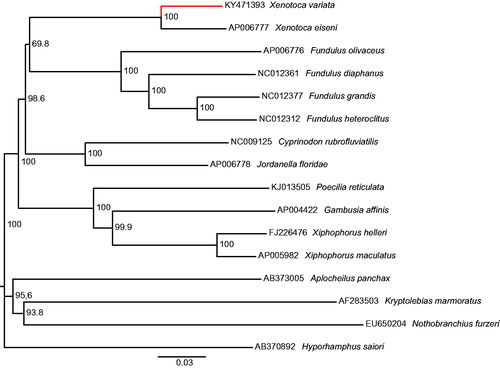
The circular mitogenome of Xenotoca variata (GenBank accession: KY471393) consists of 37 genes in 16,511bp (13 protein-coding genes, 2 rRNA genes and 22 tRNA genes) with 12 intergenic spacer sequences (of 1–37bp). The base composition of the genome was as follow: A = 28.7%, C = 26.8%, G = 15.3% and T = 29.2% (GC-rich = 42.1%). Comparing with X. eiseni, the most related of the mitogenomes included in Genbank (), an identity of 91.1% was founded and the duplication of trna-met gene in X. eiseni is absent in X. variata. The number of nucleotide differences between both mitogenomes is 1 206bp corresponding to 7.3% of divergence. The complete mitochondrial genome of X. variata provides relevant information to posterior genetic and evolutionary studies of Xenotoca genus and the Goodeidae family.
Acknowledgments
We thank the Supercomputing Center of Galicia (CESGA) for the informatics resources facilities.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data. Available from: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- Bean TH. 1887. Descriptions of five new species of fishes sent by Prof. A. Dugès from the province of Guanajuato, Mexico. Proc U S Nat Mus. 10:370–375.
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30:2114–2120.
- Corona-Santiago DK, Domínguez-Domínguez O. 2013. Systematik der Hochlandkärplinge. In: Kempkes M, Köck M, Stawikouski R, editors. Beiträge zur Biologie und zum Artenschutz der Hochlandkärpflinge. Germany: Westarp Wissenschaften; p 13–28.
- Domínguez-Domínguez O. 2008. Filogeografía de Zoogoneticus quitzeoensis, Xenotoca variata y Alloophorus robustus (Cyprinodontiformes: Goodeidae) en el centro de México: Implicaciones taxonómicas y de conservación [Dissertation]. Mexico: Instituto de Biología, Universidad Autónoma de México.
- Domínguez-Domínguez O, Pedraza-Lara C, Gurrola-Sánchez N, Pérez-Rodríguez R, Israde-Alcántara I, Garduño Monroy VH, Doadrio I, Pérez-Ponce De Léon G, Brooks DR. Historical biogeography of the Goodeinae (Cyprinodontiforms). In: Uribe MC, Grier HJ, editors. 2010. Viviparous fishes II. Homestead. Florida: New Life Publications; p 13–30.
- Faircloth BC, Glenn TC. 2012. Not all sequence tags are created equal: designing and validating sequence identification tags rogust to indels. PLoS One. 7:e42543.
- Fisher S, Barry A, Abreu J, Minie B, Nolan J, Delorey TM, Young G, Fennell TJ, Allen A, Ambrogio L, et al. 2011. A scalable, fully automated process for construction of sequence-ready human exome targeted capture libraries. Genome Biol. 12:R1.
- Fitzsimons JM. 1972. A revision of two genera of Goodeid fishes (Cyprinodontiformes, Osteichthyes) from the Mexican Plateau. Copeia. 4:728–756.
- Iwasaki W, Fukunaga T, Isagozawa R, Yamada K, Maeda Y, Satoh TP, Sado T, Mabuchi K, Takeshima H, Miya M, Nishida M. 2013. MitoFish and MitoAnnotator: a mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol Biol Evol. 30:2531–2540.
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30:3059–3066.
- Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, He G, Chen Y, Pan Q, Liu Y, et al. 2012. Soapdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 1:18.
- Matías-Garcia C. Conducta, conflicto sexual y especiación. In: Martínez-Gómez M, Velázquez-Moctezuma J, editors. 1998. Bases neurobiológicas y ecológicas de la conducta. Mexico: UAT, UAM, UNAM, UV.
- Moyaho A, Macías-Garcia C, Manjarrez J. 2004. Predation risk is associated with the geographic variation of a sexually selected trait in a viviparous fish, Xenotoca variata. J Zool. 262:265–270.
- Ritchie MG, Hamil RM, Graves JA, Magurran AE, Webb SA, Macías GC. 2007. Sexual differentiation: population genetic divergence and sexual dimorphism in Mexican goodeid fish. J Evol Biol. 20:2048–2055.
- Schattner O, Brooks AN, Lowe TM. 2005. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33:W686–W689.