Abstract
Betta pi is the largest species of mouthbrooding fighting fish, while B. splendens is a globally ornamental bubble nesting fish. Complete mitochondrial genomes (mitogenomes) of wild individuals of B. pi and B. splendens were determined. The mitogenome sequences were 16,521 and 16,980 base pair in length, containing 37 genes with gene order identical to most teleost mitogenomes. Overall A + T content was 57.72% for B. pi and 61.92% for B. splendens. Phylogenetic analysis showed that B. pi and B. splendens were highly supported monophyletic clades. Our results will facilitate further genetic studies, including mitochondrial variations and population structure of fighting fishes.
Fighting fish in the genus Betta comprise the largest group in Anabantoidei. They are separated into two groups as mouthbrooders and bubble nesting (Ruber et al. Citation2004; Chailertrit et al. Citation2014). Betta pi is the largest mouthbrooding fighting fish species, while B. splendens is a globally popular ornamental bubble nesting fish with brilliant and striking colour variations. There are many fighting fish in local aquariums and pet shops but through loss of their natural habitat wild populations are in decline. The study of genetic diversity in fighting fishes thus provides important information for prospective breeding and conservation management. Here, we determined complete mitochondrial genomes (mitogenomes) of B. pi and B. splendens collected from Narathiwat (6.4255°N, 101.8253°E) (No. KUMF6447) and Pathum Thani Provinces (14.0208°N, 100.5250°E) (No. KUMF6446), respectively, and stored in Kasetsart University Museum of Fisheries (Natural History). Whole genomic DNA was extracted in accordance with the standard salting-out protocol (Supikamolseni et al. Citation2015). Polymerase chain reaction (PCR) amplifications were performed using universal mitogenome primers (Mauro et al. Citation2004), and specific primers for both Betta species were developed to amplify the remaining parts of the genome. All PCR products were sequenced by the DNA sequencing service of First Base Laboratories Sdn Bhd (Selangor, Malaysia), and annotation was then performed following Srikulnath et al. (Citation2012).
The complete mitogenome sequences consisted of 16,521 bp for B. pi (GenBank accession no. AB920288) and 16,980 bp for B. splendens (AB571120). Both mitogenomes contained 37 genes, and a control region (). Gene arrangement patterns were identical to those of teleosts (Miya et al. Citation2013). Four conserved sequence blocks: CSB-D, CSB-1, CSB-2 and CSB-3 found in the control region of teleost mitogenomes were also found in B. pi and B. splendens (Lee and Kocher Citation1995). However, 19 bp insertion was observed in CSB-1 of B. pi. A tandem repeat was identified at positions 15,681–15,724 bp in B. pi. Moreover, in B. splendens, two tandem repeats were identified at positions 15,793–16,150 bp and 16,906–16,971 bp. This differed from the control region structure of the B. splendens mitogenome (KR527219) which had three tandem repeats (Song et al. Citation2016), suggesting that the control region in B. splendens varies between individuals and might be applicable for population genetic study. Comparison of overall nucleotide diversity among B. pi, B. splendens (AB571120) and B. splendens (KR527219) mitogenomes was determined at 13.92%. Both B. splendens individuals showed 0.64% nucleotide divergence, suggesting intra-specific sequence diversity. The phylogenetic tree was constructed based on concatenated twelve protein-coding genes without ND6 of 16 teleosts with fighting fishes, using Bayesian inference with MrBayes version 3.2.6 (Huelsenbeck and Ronquist Citation2001). B. splendens (AB571120) and B. splendens (KR527219) as a sister clade formed a monophyletic group with B. pi. The fighting fish clade was close to the Macropodus clade, confirming results from previous studies (Ruber et al. Citation2004) (). These complete mitogenomes will increase understanding of the evolutionary processes and diversification of the fighting fish group and facilitate more thorough analyses.
Figure 1. Phylogenetic relationships among concatenated mitochondrial twelve protein-coding genes without ND6 sequences of 19 mitochondrial genomes including Eleotris acanthopoma as the outgroup inferred using Bayesian inference analysis. The complete mitochondrial genome sequence was downloaded from GenBank. The accession number is indicated in parentheses after the scientific name of each species. Support values at each node are Bayesian posterior probabilities. The branch-lengths represent number of nucleotide substitutions per site.
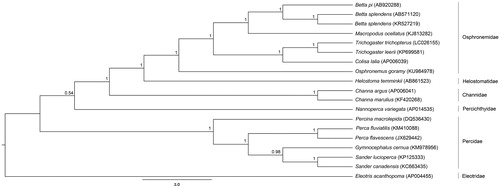
Table 1. Comparison of nucleotide length, AT content, start codon, stop codon and nucleotide sequence diversity between the three complete mitochondrial genomes of Betta pi (AB920288), B. splendens (AB571120) and B. splendens (KR527219).
Acknowledgements
The authors would like to thank Akarapong Swatdipong and Sahabhop Dokkaew (Kasetsart University, Thailand), and Nonn Panitvong (Siamensis.org) for sample collection.
Disclosure statement
The authors report no conflict of interest and are responsible for the content and writing of this article. Animal care and all experimental procedures were approved by the Animal Experiment Committee, Kasetsart University, Thailand (approval no. ACKU01157), and conducted in accordance with the Regulations on Animal Experiments at Kasetsart University.
Additional information
Funding
References
- Chailertrit V, Swatdipong A, Peyachoknagul S, Salaenoi J, Srikulnath K. 2014. Isolation and characterization of novel microsatellite markers from Siamese fighting fish (Betta splendens, Osphronemidae, Anabantoidei) and their transferability to related species, B. smaragdina and B. imbellis. Genet Mol Res. 13:7157–7162.
- Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 17:754–755.
- Lee WJ, Kocher TD. 1995. Complete sequence of a sea lamprey (Petromyzon marinus) mitochondrial genome: early establishment of the vertebrate genome organization. Genetics. 139:873–887.
- Mauro DS, David JJG, Oommen VO, Wilkinson M, Zardoya R. 2004. Phylogeny of caecilian amphibians (Gymnophiona) based on complete mitochondrial genomes and nuclear RAG1. Mol Phylogenet Evol. 33:413–427.
- Miya M, Friedman M, Satoh TP, Takeshima H, Sado T, Iwasaki W, Yamanoue Y, Nakatani M, Nakatani K, Inoue JG, et al. 2013. Evolutionary origin of the Scombridae (Tunas and Mackerels): members of a paleogene adaptive radiation with 14 other pelagic fish families. PLoS One. 8:e73535
- Ruber L, Britz R, Tan HH, Ng PKL, Zardoya R. 2004. Evolution of mouthbrooding and life-history correlates in the fighting fish genus Betta. Evolution. 58:799–813.
- Song YN, Xiao GB, Li JT. 2016. Complete mitochondrial genome of the Siamese fighting fish (Betta splendens). Mitochondrial DNA A DNA Mapp Seq Anal. 27:4580–4581.
- Srikulnath K, Thongpan A, Suputtitada S, Apisitwanich S. 2012. New haplotype of the complete mitochondrial genome of Crocodylus siamensis and its species-specific DNA markers: distinguishing C. siamensis from C. porosus in Thailand. Mol Biol Rep. 39:4709–4717.
- Supikamolseni A, Ngaoburanawit N, Sumontha M, Chanhome L, Suntrarachun S, Peyachoknagul S, Srikulnath K. 2015. Molecular barcoding of venomous snakes and species-specific multiplex PCR assay to identify snake groups for which antivenom is available in Thailand. Genet Mol Res. 14:13981–13997.
