Abstract
The whole mitochondrial genome of a small cyprinid freshwater fish Pectenocypris sp. collected from Serkap River, Central Sumatra, Indonesia was sequenced. This mitochondrial genome consisted of 16,589 bp and included 37 genes in the same order as in many other vertebrates including the human. Phylogenetic analysis suggested that this taxon clusters with Boraras maculatus among several Rasbora species.
Pectenocypris (Actinopterygii: Cyprinidae) consists of four known freshwater fish species which are distributed in Sumatra and Borneo Islands: P. balaena, P. korthausae, P. micromysticetus and P. nigra. They are phytoplankton filter feeders (Rainboth Citation1991) but also feed on zooplankton (Kottelat Citation1982; Roberts Citation1989). All the species are small in size (<45 mm in standard length) and typically occur in acidic freshwaters. They have slender body, numerous and extraordinary extended gill rakers and a unique shaped lower pharyngeal jaw (Kottelat Citation1982; Roberts Citation1989; Tan and Kottelat Citation2009; Wibowo et al. Citation2016).
We conducted field survey at freshwaters in Central Sumatra and found possibly new species assignable to Pectenocypris. An adult individual of the undescribed Pectenocypris species with a standard length 31.1 mm was collected in peat waters of the Serkap River system in the Riau Province near Pelalawan, Central Sumatra (geographic coordinate: 00°34'42”N, 102°39'17”E) in 2013. The whole body specimen was deposited to the Museum Zoologicum Bogoriense (MZB), Bogor under the catalogue number MZB 22148. A small portion of muscle tissue was excised under the dorsal fin and preserved in ethanol for subsequent DNA extraction by Genomic DNA Mini Kit (Geneaid/ New Taipei City). Mitochondrial DNA was purified by digesting the linear nuclear DNA with the exonuclease V under the Mseek protocol (Jayaprakash et al. Citation2015) and then sequenced using the NEXTflexTM Rapid DNA-Seq kit (Bioo Scientific/ Texas) with the Illumina NextSeq platform. This resulted in a single, circular DNA sequence which was then blasted against the Mitofish database (Iwasaki et al. Citation2013) for the confirmation of a fish mitochondrial DNA.
The complete mitochondrial DNA sequence of Pectenocypris sp. thus determined (16,589 bp; INSD database accession number LC337232) consisted of 37 genes for 13 protein subunits, 22 tRNAs, and two rRNAs together with a major non-coding region in the same order as in human (Anderson et al. Citation1981) and many other vertebrates. All protein genes had an ATG start codon except for the cytochrome oxidase subunit 1, and NADH dehydrogenase subunits 4L and 5 genes, which started with GTG, GTG and ATA, respectively. Seven protein genes were terminated with the TAA stop codon and the remaining six genes required polyadenylation for the establishment of stop codons in mRNAs. All tRNA genes can be folded into the standard cloverleaf secondary structures (Kumazawa and Nishida Citation1993).
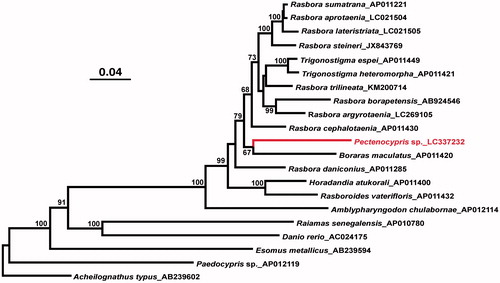
This is the first complete mitochondrial genome sequence from genus Pectenocypris. The phylogenetic tree () suggested that Rasbora is not monophyletic in relation to genera Trigonostigma, Pectenocypris, and Boraras, which is in agreement with earlier molecular studies (Rüber et al. Citation2007; Britz et al. Citation2009; Tang et al. Citation2010). It was also suggested that contrary to the earlier work (e.g. Tang et al. Citation2010) Pectenocypris sp. is more closely related to Boraras maculatus (dwarf rasbora) than to other rasborine cyprinids examined although the bootstrap probability for this relationship was not very high (67%; ).
Figure 1. A maximum likelihood tree illustrating the phylogenetic position of Pectenocypris sp. among other rasborine cyprinids. The maximum likelihood analysis was conducted using concatenated amino acid sequences of 13 mitochondrial protein genes (3,813 sites) and Garli v2.0 (Zwickl Citation2017) under the mtREV + IG substitution model. Numbers at each node are bootstrap probabilities by 500 replications shown only when they are 50% or larger. INSD accession numbers of mitogenomic sequences for each taxon are shown along with the taxon name.
Acknowledgements
We thank Drs. Satoshi Honda and Kaoru Ishii of Inland Fisheries Resources Development and Management Department (IFRDMD) and Southeast Asian Fisheries Development Center (SEAFDEC), and Mr. Atsushi Saga and Ms. Yo Soma of Marino Forum 21 for providing and arranging the SEAFDEC short-term training for DA at Nagoya City University. Our gratitude is extended to the Laboratory of Molecular Ecology of Research Institute for Inland fisheries where a part of our molecular analysis was done.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. 1981. Sequence and organization of the human mitochondrial genome. Nature. 290:457–465.
- Britz R, Conway KW, Rüber L. 2009. Spectacular morphological novelty in a miniature cyprinid fish, Danionella dracula n. sp. Proc R Soc Lond B. 276:2179–2186.
- Iwasaki W, Fukunaga T, Isagozawa R, Yamada K, Maeda Y, Satoh TP, Sado T, Mabuchi K, Takeshima H, Miya M, et al. 2013. MitoFish and MitoAnnotator: a mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol Biol Evol. 30:2531–2540.
- Jayaprakash AD, Benson EK, Gone S, Liang R, Shim J, Lambertini L, Toloue MM, Wigler M, Aaronson SA, Sachidanandam R. 2015. Stable heteroplasmy at the single-cell level is facilitated by intercellular exchange of mtDNA. Nucleic Acids Res. 43:2177–2187.
- Kottelat M. 1982. A small collection of fresh-water fishes from Kalimantan, Borneo, with descriptions of one new genus and three new species of Cyprinidae. Rev Suisse Zool. 89:419–437.
- Kumazawa Y, Nishida M. 1993. Sequence evolution of mitochondrial tRNA genes and deep-branch animal phylogenetics. J Mol Evol. 37:380–398.
- Rainboth WJ. 1991. Cyprinids of South East Asia. In: Winfield IJ and Nelson JS, editor. Cyprinid fishes: systematics, biology and exploitation. London (UK): Chapman and Hall; p. 156–210.
- Roberts TR. 1989. The freshwater fishes of Western Borneo (Kalimantan Barat, Indonesia). Mem Calif Acad Sci. 14:1–210.
- Rüber L, Kottelat M, Tan HH, Peter KN, Britz R. 2007. Evolution of miniaturization and the phylogenetic position of Paedocypris, comprising the world’s smallest vertebrate. BMC Evol Biol. 7:38.
- Tan HH, Kottelat M. 2009. The fishes of Batang Hari drainage, Sumatra, with description of six new species. Ichthyol Explor Freshwaters. 20:13–69.
- Tang KL, Agnew MK, Hirt MV, Sado T, Schneider LM, Freyhof J, Sulaiman Z, Swartz E, Vidthayanon C, Miya M, et al. 2010. Systematics of the subfamily Danioninae (Teleostei: Cypriniformes: Cyprinidae). Mol Phylogenet Evol. 57:189–214.
- Wibowo A, Ahnelt H, Kertamihardja ES. 2016. Pectenocypris nigra, a new danionine species (Teleostei: Cyprinidae: Danioninae) from Sumatara (Indonesia). Acta Biologica Turcica. 29:137–142.
- Zwickl DJ. 2017. Genetic Algorithm for Rapid Likelihood Inference. Version 2.0. [Internet]. [accessed 2017 Feb 27]. http://www.bio.utexas.edu/faculty/antisense/garli/garli.html
