Abstract
Solanum stoloniferum is a wild tuber-bearing species belonging to Solanaceae family. The complete chloroplast genome of S. stoloniferum was constituted by de novo assembly using a small amount of whole genome sequencing data. The chloroplast genome of S. stoloniferum was the circular DNA molecule with a length of 155,567 bp and consisted of 86,007 bp of large single copy, 18,374 bp of small single copy, and 25,593 bp of a pair of inverted repeat regions. A total of 158 genes were annotated including 105 protein-coding genes, 45 tRNA genes, and eight rRNA genes. Maximum likelihood phylogenetic analysis with 25 Solanaceae species revealed that S. stoloniferum is the most closely grouped with S. tuberosum.
Solanum stoloniferum, a wild tuber-bearing tetraploid species originating from Mexico is a relative to the cultivated potato, S. tuberosum (Hawkes Citation1990; Pendinen et al. Citation2008). It was identified to be a source of resistance to late blight and potato virus Y for potato breeding (Flis et al. Citation2005; Valkonen et al. Citation2008; Wang et al. Citation2008). However, the species is sexually incompatible with S. tuberosum due to different endosperm balance numbers (EBNs) with EBN values of 2 and 4 in S. stoloniferum and S. tuberosum, respectively, although both species are tetraploid (Brown Citation1988; Singsit and Hanneman Citation1991; Ortiz and Ehlenfeldt Citation1992; Cho et al. Citation1997). So, the wild species is difficult to be applied to potato breeding and somatic hybridization could be one of the solutions to overcome sexual barriers for interspecific gene transfer (Bidani et al. Citation2007; Nouri-Ellouz et al. Citation2016) and importance to identify chlorotype with information of chloroplast genome sequences in potato breeding program has increased (Chen et al. Citation2013, Citation2016; Cho and Park Citation2016; Cho et al. Citation2016; Molnár et al. Citation2017).
The S. stoloniferum (PI160224) was provided by Highland Agriculture Research Institute, South Korea. An Illumina paired-end (PE) genomic library was constructed with total genomic DNA according to the PE standard protocol (Illumina, San Diego, CA) and sequenced using an Illumina HiSeq2000 at Macrogen (http://www.macrogen.com/kor/). Low-quality bases with raw scores of 20 or less were removed and approximately 5.3 Gbp of high-quality of PE reads were assembled by a CLC genome assembler (CLC Inc, Arhus, Denmark) (Kim et al. Citation2015). The reference chloroplast genome sequence of S. commersonii (KM489054, Cho et al. Citation2016) was used to retrieve principal contigs representing the chloroplast genome from the total contigs using Nucmer (Kurtz et al. Citation2004). The representative chloroplast contigs were arranged in order based on BLASTZ analysis (Schwartz et al. Citation2003) with the reference sequence and connected to a single draft sequence by joining overlapping terminal sequences. DOGMA (Wyman et al. Citation2004) and BLAST searches were used to predict chloroplast genes.
The complete chloroplast genome of S. stoloniferum (GenBank accession no. MF471373) was 155,567 bp in length including 25,593 bp inverted repeats (IRa and IRb) regions separated by small single copy (SSC) region of 18,374 bp and large single copy (LSC) region of 86,007 bp with the typical quadripartite structure of most plastids, and the structure and gene features were typically identical to those of higher plants. A total of 158 genes with an average size of 584.4 bp were annotated including 105 protein-coding genes with an average size of 766.6 bp, 45 tRNA genes, and eight rRNA genes. An overall GC content was 37.87%.
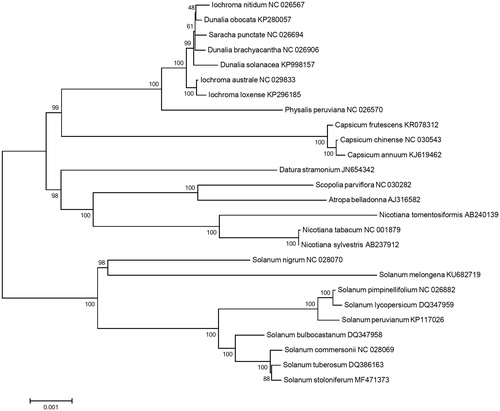
Phylogenetic analysis was performed using chloroplast coding sequences of S. stoloniferum and 25 published species in Solanaceae family by a maximum likelihood method in MEGA 6.0 (Tamura et al. Citation2013). According to the phylogenetic tree, S. stoloniferum belonged to the same clade in Solanum species as expected and interestingly it was most closely grouped with S. tuberosum ().
Disclosure statement
The author reports no conflicts of interest. The author alone is responsible for the content and writing of this article.
Additional information
Funding
References
- Bidani A, Nouri-Ellouz O, Lakhoua L, Sihachakr D, Cheniclet C, Mahjoub A, Drira N, Gargouri-Bouzid R. 2007. Interspecific potato somatic hybrids between Solanum berthaultii and Solanum tuberosum L. showed recombinant plastome and improved tolerance to salinity. Plant Cell Tiss Organ Cult. 91:179–189.
- Brown CR. 1988. Characteristics of 2N pollen producing triploid hybrids between Solanum stoloniferum and cultivated diploid potatoes. Am Potato J. 65:75–84.
- Chen L, Guo X, Wang H, Xie C, Cai X, He L, Zhou J, Liu J. 2016. Tetrasomic inheritance pattern of the pentaploid Solanum chacoense (+) S. tuberosum somatic hybrid (resistant to bacterial wilt) revealed by SSR detected alleles. Plant Cell Tissue Org Cult. 127:315–323.
- Chen L, Guo X, Xie C, He L, Cai X, Tian L, Song B, Liu J. 2013. Nuclear and cytoplasmic genome components of Solanum tuberosum + S. chacoense somatic hybrids and three SSR alleles related to bacterial wilt resistance. Theor Appl Genet. 126:1861–1872.
- Cho HM, Kim-Lee HY, Om YH, Kim JK. 1997. Influence of endosperm balance number (EBN) in interploidal and interspecific crosses between Solanum tuberosum dihaploids and wild species. Korean J Breed. 29:154–161.
- Cho K-S, Cheon K-S, Hong S-Y, Cho J-H, Im J-S, Mekapogu M, Yu Y-S, Park T-H. 2016. Complete chloroplast genome sequences of Solanum commersonii and its application to chloroplast genotype in somatic hybrids with Solanum tuberosum. Plant Cell Rep. 35:2113–2123.
- Cho K-S, Park T-H. 2016. Complete chloroplast genome sequence of Solanum nigrum and development of markers for the discrimination of S. nigrum. Hortic Environ Biotechnol. 57:69–78.
- Flis B, Henning J, Strzelczyk-Żyta D, Gebhardt C, Marczewski W. 2005. The Ry-fsto gene from Solanum stoloniferum from extreme resistant to Potato virus Y maps to potato chromosome XII and is diagnosed by PCR marker GP122718 in PVY resistant potato cultivars. Mol Breed. 15:95–101.
- Hawkes JG. 1990. The potato: evolution, biodiversity and genetic resources. London, UK: Belhaven Press.
- Kim K, Lee SC, Lee J, Lee H, Joh HJ, Kim NH, Park HS, Yang TJ. 2015. Comprehensive survey of genetic diversity in chloroplast genomes and 45S nrDNAs within Panax ginseng species. PLoS One. 10:e0117159.
- Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. 2004. Versatile and open software for comparing large genomes. Genome Biol. 5:R12.
- Molnár I, Besenyei E, Thieme R, Thieme T, Aurori A, Baricz A, Banciu HL, Rakory-Tican E. 2017. Mismatch repair deficiency increases the transfer of antibiosis and antixenosis properties against Colorado potato beetle in somatic hybrids of Solanum tuberosum + S. chacoense. Pest Manage Sci. 73:1428–1437.
- Nouri-Ellouz O, Triki MA, Jbir-Koubaa R, Louhichi A, Charfeddine S, Drira N, Gargouri-Bouzid R. 2016. Somatic hybrids between potato and S. berthaultii show partial resistance to soil-borne fungi and potato virus Y. J Phytopathol. 164:485–496.
- Ortiz R, Ehlenfeldt MK. 1992. The importance of endosperm balance number in potato breeding and the evolution of tuber-bearing Solanum species. Euphytica. 60:105–113.
- Pendinen G, Gavrilenko T, Jiang J, Spooner DM. 2008. Allopolyploid speciation of the Mexican tetraploid potato species Solanum stoloniferum and S. hjertingii revealed by genomic in situ hybridization. Genome. 51:714–720.
- Schwartz S, Kent WJ, Smit A, Zhang Z, Baertsch R, Hardison RC, Haussler D, Miller W. 2003. Human-mouse alignments with BLASTZ. Genome Res. 13:103–107.
- Singsit C, Hanneman RE Jr. 1991. Rescuing abortive inter-EBN potato hybrids through double pollination and embryo culture. Plant Cell Rep. 9:475–478.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 30:2725–2729.
- Valkonen JPT, Wiegmann K, Hämäläinen JH, Marczewski W, Watanabe KN. 2008. Evidence for utility of the same PCR-based markers for selection of extreme resistance to Potato virus Y controlled by Rysto of Solanum stoloniferum derived from different sources. Ann Appl Biol. 152:121–130.
- Wang M, Allefs S, van den Berg RG, Vleeshouwers VGAA, van der Vossen EAG, Vosman B. 2008. Allele mining in Solanum: conserved homologues of Rpi-blb1 are identified in Solanum stoloniferum. Theor Appl Genet. 116:933–943.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20:3252–3255.