Abstract
Bat populations are declining worldwide. Accurate identification is essential to promote species’ conservation. However, minimal morphological differences and a high rate of cryptic species make identification difficult, unless voucher specimens are kept, a controversial issue today. The objective of this work was to standardize a method of extracting non-lethal DNA using bats’ uropatagium micro-tissue, aiming the molecular identification of species that occur in the region of Maringá PR. The method standardized was efficient, and does not cause serious damage to bats. For future field studies, collection of micro-tissue and morphometry of the specimens will be sufficient for accurate identification.
Introduction
Chiroptera presents over 1300 recognized species (Tsang et al. 2016) and although they perform important ecological functions (Boyles et al. Citation2011), at least 16% of the species are threatened (Voigt and Kingston 2016, IUCN 2017). Among the measures in the reversion of this is a correct identification of the species, mainly performed through morphological analysis (Francis et al. Citation2010), or either by acoustic analysis of echolocation, which presents practical problems (Rydell et al. Citation2017), or by molecular techniques (Clare Citation2011; Pavan and Marroig Citation2016).
Most species present minimal morphological differences, overlapping measurements (Miranda et al. Citation2011) and a high rate of cryptic species (Clare et al. Citation2007), highlighted by molecular studies (Dool et al. Citation2016; Gager et al. Citation2016). Thus, field identifications are questionable unless voucher specimens are kept, which is oftentimes hampered by environmental licences or ethical concerns (Wilson et al. Citation2014). It is still a controversial issue in modern biology (Russo et al. Citation2017) and raises concerns about unnecessary collections of organisms (Corthals et al. Citation2015), having as an aggravating that vouchers could in many cases be safely replaced with images and molecular studies (Corthals et al. Citation2015; Raupach et al. Citation2016). The DNA barcode has been widely used as a fast and accurate tool in the identification and differentiation of the species (Clare et al. Citation2011; Wilson et al. Citation2014).
In some studies, non-lethal methods for molecular research have been used, such as fecal samples, buccal swab, blood (Walker et al. Citation2016) and the wing and tail membrane (Faure et al. Citation2009; Wilson et al. Citation2014). Uropatagium tissue has been recommended for collection because it heals quickly and it provides a high quantity of DNA (Faure et al. Citation2009). Many field studies pierce these membranes to mark the animal by discarding the surplus micro-tissue. The objective of this work was to standardize a method of extracting non-lethal DNA from a bats’ uropatagium micro-tissue, aiming the molecular identification of species that occur in the region of Maringá PR.
Methodology
Collection of material
The collections were carried out by the Grupo de Estudos em Ecologia de Mamíferos e Educação Ambiental (GEEMEA) in fragments of Atlantic Forest in the city of Maringá, Paraná, Brazil (23°25′58″S 51°58′06″W). Collections were performed using mist. Individuals were measured and identified according to the criteria of Gregorin and Taddei (Citation2002). The tissue (1 mm2) was extracted from the uropatagium using a disposable biopsy punch (Disposable Biopsy Punch, 1 mm – Miltex®), afterwards it was stored in a microtube with absolute ethanol. The tubes were kept at −20 °C until DNA extraction.
Uropatagium tissues were collected from 50 specimens, which were released and observed flying without difficulties. The bats captured were attributed to 13 species (). Specimens were collected under a scientific collecting permit (Sisbio 55121-2, process 3097240916).
Table 1. Number of samples collected by Chiroptera species in the northwestern region of Paraná.
Extraction of DNA
The DNA was extracted with the ReliaPrep ™ column extraction kit (ReliaPrep ™ gDNA Tissue Miniprep System – Promega), with three different protocols: (I) 10 extractions of DNA from uropatagium were performed according to the manufacturer’s instructions; (II) 10 extractions of DNA from uropatagium were performed. Initially the tissue was rehydrated in TE (TRIS HCl pH 8.0 10 mM/EDTA pH 8.0 1 M) for 1 h, then the steps recommended by the manufacturer were performed, but the DNA was re-suspended in only 50 μL of Nuclease-Free Water; and (III) 30 individuals were used in extraction III, following the same steps as extraction II, but the tissue sample was triturated with slide prior to incubation at 56 °C for 3 h.
PCR and DNA sequencing
Partial sequences of the mitochondrial cytochrome C oxidase I gene (COI) were obtained with the primers described by Ivanova et al. (Citation2007). For confirming the success of the extraction, samples that did not amplify with COI were subjected to amplification using primers from part of the Histone H3 nuclear gene (Colgan et al. Citation1998). Finally, samples that did not amplify with the Histone H3 were discarded.
The PCR reactions (25 μL) were carried out containing Tris-KCl (20 mM of Tris-HCl, pH 8.4 and 50 mM of KCl), 1.5 mM of MgCl2, 2.5 mM of each primer, 0.1 mM of each dNTP, 1 μL Taq of DNA polymerase, mold DNA and water. Since it was not possible to quantify the DNA extraction by virtue of their small amount, two different volumes of template DNA were tested: the first PCR (1) with 2 μL and the second (2) with 5 μL.
The temperatures used in the PCR followed an initial cycle at 94 °C, 1 min; five cycles at 94 °C, 30 s, 50 °C, 40 s, and 72 °C, 1 min; followed by 35 cycles at 94 °C, 30 s, 55 °C (COI) – 58 °C (Histone H3), 40 s and 72 °C, 1 min, followed by a final extension for 10 min at 72 °C. Amplicons of the COI were purified according to Rosenthal et al. (Citation1993), and these were subjected to BigDye Terminator Cycle (Foster City, CA) sequencing and DNA sequencing was performed using a Applied Biosystems 3730XL(Carlsbad, CA), both according to manufacturer’s instructions.
Data analysis
The genetic distance matrix, sequences alignment and model selection were performed in the MEGA 7 program (Kumar et al. Citation2016). A phylogenetic network was constructed using the NeighborNet method (Bryant and Moulton Citation2004), with SplitsTree4 (Huson and Bryant Citation2006). The phylogenetic trees were constructed using raxmlGUI (Silvestro and Michalak 2012) and MrBayes 3.2 (Ronquist et al. 2012), considering the statistical methods of maximum likelihood and Bayesian inference, respectively. The BLAST method was used to species identification using COI (Ross et al. Citation2008).
Results
The samples extracted in the I and II protocols showed no amplification. However, the III protocol (30 samples) resulted in 23 samples amplified with COI, and 70% were obtained from PCR reaction with 2 μL of template DNA. Seven samples that did not amplify with the COI were tested with Histone H3; of these, three amplified, confirming the success of the extraction.
Partial sequences of COI with 595 bp were obtained from 23 specimens, distributed in 13 species, with 21 different haplotypes. Specimens attributed to different Artibeus species shared haplotypes (specimens 224 and 217; specimens 382 and 207).
The interspecific variation in Artibeus was 0.72%. Some species collected showed similarity of 100% with species deposited in GenBank ().
Table 2. Identification of morphologic and identification of the species using similarity analysis with the BLAST algorithm, COI sequences available in GenBank and of COI sequences obtained from DNA extracted from the bats’ uropatagium micro-tissue (e-value <0.0).
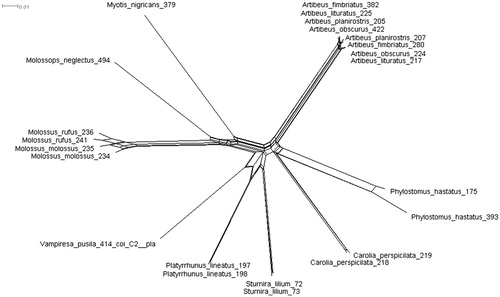
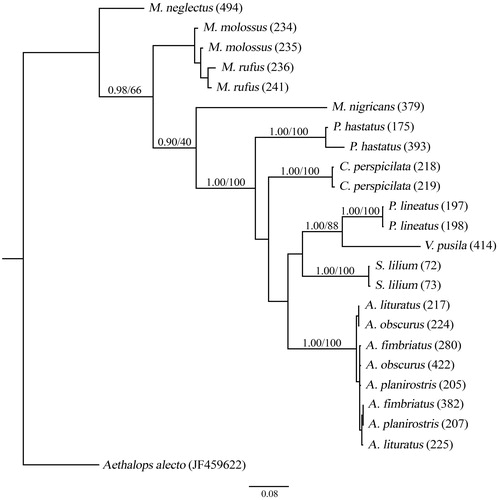
The NeighborNet phylogenetic network and the phylogenetic tree showed that individuals of the same species were grouped, with the exception of individuals of Artibeus species ( and ).
Discussion
The results of this study showed that it is possible to successfully carry out DNA extraction from uropatagium micro-tissue modifying the manufacturer’s protocol, reducing the risks for the animal. Many studies use wing membrane tissue for DNA extraction; however, multiple biopsies are needed from the same animal, aiming to increase the tissue mass for extraction (Vonhof et al. Citation2008). This procedure causes pain and increases the bleeding potential.
The III protocol was the only one that allowed to amplify the two genes tested. Rehydration, triturated of the sampled material and increase in incubation time may have facilitated the extraction process, since studies have already demonstrated that in membrane extractions of flight it is common that the tissue is not completely digested (Faure et al. Citation2009), besides most of the samples were amplified with less amount of template DNA.
The initial identification of specimen 494 was that of Molossops neglectus. However, according to the COI gene, the specimen presented greater genetic similarity to M. temminckii, a species that is also registered in Paraná (Miretzki and Margarido Citation1999). M. neglectus and M. temminckii are supported as sister species (Peters et al. Citation2002), justifying the high genetic similarity evidenced by the COI gene. This fact reinforces the thesis of errors in the identification of specimens at the capture site.
The specimens attributed to Artibeus fimbriatus, A. obscurus and A. planirostris presented 100% similarity to A. lituratus, which was expected since specimens attributed to different Artibeus species shared haplotypes. Several studies with bats indicate that the average intraspecific variation does not exceed 2% (Bradley and Baker Citation2001; Francis et al. Citation2010; Clare Citation2011; Clare et al. Citation2011).
Artibeus presents difficulties in species identification based on morphological characters. There are overlapping measurements for A. fimbriatus, A. obscurus and A. planirostris and A. lituratus, and because they are morphologically very similar, they are commonly confused (Araújo and Langguth Citation2010). Thus, both the specimens used in this work and the sequences of the specimens that were deposited in GenBank may have been misidentified. Of the seven specimens that were not conclusively identified, six were Artibeus.
Excluding the Artibeus specimens, 93.3% of the sequenced samples were conclusively identified. In studies using barcode DNA in bats, 96.6% of the individuals sampled were correctly identified (Borisenko et al. Citation2008). Therefore, we conclude that DNA barcode is efficient in identifying bat species in the neotropical region.
It is important to emphasize that for the extraction of DNA, it was not necessary to euthanize the animal, and it was possible to amplify a nuclear marker, indicating that other markers can be used to solve complex questions. In future field studies, only the collection of micro-tissue, photographic images and morphometry of the specimens will be sufficient for correct identification, contributing to the reduction of ethical problems in research and animal welfare.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Araújo P, Langguth A. 2010. Caracteres distintivos das quatro espécies de grandes Artibeus (Phyllostomidae) de Paraíba e Pernambuco, Brasil. Chiroptera Neotrop. 16:715–722.
- Borisenko AV, Lim BK, Ivanova NV, Hanner RH, Hebert PDN. 2008. DNA barcoding in surveys of small mammal communities: a field study in Suriname. Mol Ecol Resour. 8:471–479.
- Boyles JG, Cryan PM, McCracken GF, Kunz TH. 2011. Conservation. Economic importance of bats in agriculture. Science. 332:41–42.
- Bradley RD, Baker RJ. 2001. A test of the genetic species concept: cytochrome-b sequences and mammals. J Mammal. 82:960–973.
- Bryant D, Moulton V. 2004. Neighbor-Net: an agglomerative method for the construction of phylogenetic networks. Mol Biol Evol. 21:255–265.
- Clare EL. 2011. Cryptic species? Patterns of maternal and paternal gene flow in eight neotropical bats. PLoS One. 6:e21460.
- Clare EL, Lim BK, Engstrom MD, Eger JL, Hebert PDN. 2007. DNA barcoding of neotropical bats: species identification and discovery within Guyana. Mol Ecol Notes. 7:184–190.
- Clare EL, Lim BK, Fenton MB, Hebert PDN. 2011. Neotropical bats: estimating species diversity with DNA barcodes. PLoS One. 6:e22648.
- Colgan DJ, McLauchlan A, Wilson GDF, Livingston SP, Edgecombe GD, Macaranas J, Cassis G, Gray MR. 1998. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Aust J Zool. 46:419–437.
- Corthals A, Martin A, Warsi OM, Woller-Skar M, Lancaster W, Russell A, Dávalos LM. 2015. From the field to the lab: best practices for field preservation of bat specimens for molecular analyses. PLoS One. 10:e0118994.
- Dool SE, Puechmaille SJ, Foley NM, Allegrini B, Bastian A, Mutumi GL, Maluleke TG, Odendaal LJ, Teeling EC, Jacobs DS. 2016. Nuclear introns outperform mitochondrial DNA in inter-specific phylogenetic reconstruction: lessons from horseshoe bats (Rhinolophidae: Chiroptera). Mol Phylogenet Evol. 97:196–212.
- Faure PA, Re DE, Clare EL. 2009. Wound healing in the flight membranes of big brown bats. J Mammal. 90:1148–1156.
- Francis CM, Borisenko AV, Ivanova NV, Eger JL, Lim BK, Guillen-Servent A, Kruskop SV, Mackie I, Hebert PDN. 2010. The role of DNA barcodes in understanding and conservation of mammal diversity in Southeast Asia. PLoS One. 5:e12575.
- Gager Y, Tarland E, Lieckfeldt D, Ménage M, Botero-Castro F, Rossiter SJ, Kraus RHS, Ludwig A, Dechmann DKN. 2016. The value of molecular vs. morphometric and acoustic information for species identification using sympatric molossid bats. PLoS One. 11:e0150780.
- Gregorin R, Taddei VA. 2002. Chave artificial para a identificação de Molossídeos brasileiros (Mammalia, Chiroptera). Mastozool Neotrop. 9:13–32.
- Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 23:254–267.
- Ivanova NV, Zemlak TS, Hanner RH, Hebert PDN. 2007. Universal primer cocktails for fish DNA barcoding. Mol Ecol Notes. 7:544–548.
- IUCN. 2017. The IUCN Red List of Threatened Species. [accessed 2018 May 10]. http://www.iucnredlist.org.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33:1870–1874.
- Miranda JMD, Bernardi IP, Passos FC. 2011. Chave ilustrada para determinação dos morcegos da Região Sul do Brasil. Curitiba: João MD Miranda.
- Miretzki M, Margarido TCC. 1999. Morcegos da Estação Ecológica do Caiuá, Paraná (Sul do Brasil.). Chiroptera Neotrop. 5:105–108.
- Pavan AC, Marroig G. 2016. Integrating multiple evidences in taxonomy: species diversity and phylogeny of mustached bats (Mormoopidae: Pteronotus). Mol Phylogenet Evol. 103:184–198.
- Peters SL, Lim BK, Engstrom MD. 2002. Systematics of dog-faced bats (Cynomops) based on molecular and morphometric data. J Mammal. 83:1097–1110.
- Raupach MJ, Amann R, Wheeler QD, Roos C. 2016. The application of “-omics” technologies for the classification and identification of animals. Organ Div Evol. 16:1–12.
- Rosenthal A, Coutelle O, Craxton M. 1993. Large-scale production of DNA sequencing templates by microtitre format PCR. Nucleic Acids Res. 21:173–174.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542.
- Ross HA, Murugan S, Li WLS. 2008. Testing the reliability of genetic methods of species identification via simulation. Syst Biol. 57:216–230.
- Russo D, Ancillotto L, Hughes AC, Galimberti A, Mori E. 2017. Collection of voucher specimens for bat research: conservation, ethical implications, reduction, and alternatives. Mammal Rev. 47:237–246.
- Rydell J, Nyman S, Eklöf J, Jones G, Russo D. 2017. Testing the performances of automated identification of bat echolocation calls: a request for prudence – ScienceDirect. Ecol Indicat. 78:416–420.
- Silvestro D, Michalak I. 2012. RaxmlGUI: A graphical front-end for RAxML. Organisms Diversity and Evolution. 12(4):335–337.
- Tsang SM, Cirranello AL, Bates PJJ, Simmons NB. 2016. The roles of taxonomy and systematics in bat conservation. In: Voigt CC, Kingston T, editors. Bats in the anthropocene: conservation of bats in a changing world. Cham: Springer International Publishing; p. 503-538.
- Voigt CC, Kingston T. 2016. Bats in the anthropocene: conservation of bats in a changing world. Vol. 606. New York: SpringerOpen.
- Vonhof MJ, Strobeck C, Fenton MB. 2008. Genetic variation and population structure in big brown bats (Eptesicus fuscus): is female dispersal importante? J Mammal. 89:1411–1420.
- Walker FM, Williamson CHD, Sanchez DE, Sobek CJ, Chambers CL. 2016. Species from feces: order-wide identification of Chiroptera from guano and other non-invasive genetic samples. PLoS One. 11:e0162342.
- Wilson JJ, Sing KW, Halim MRA, Ramli R, Hashim R, Sofian-Azirun M. 2014. Utility of DNA barcoding for rapid and accurate assessment of bat diversity in Malaysia in the absence of formally described species. Genet Mol Res. 13:920–925.