Abstract
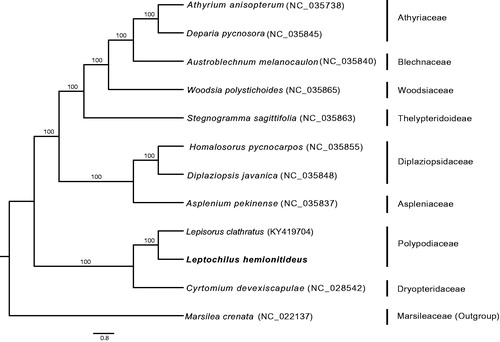
The complete chloroplast genome of Leptochilus hemionitideus was sequenced. Its length is 156,083 bp with 44.2% GC content. The genome exhibits typical quadripartite with two inverted repeat regions (24,594 bp, each) separated by a large single-copy (LSC, 81,403 bp) region and a small single-copy (SSC, 25,492 bp) region. It has 131 genes, including 87 protein-coding genes, 34 tRNA genes, eight rRNA genes and two pseudogenes. Maximum-likelihood phylogenetic tree indicated that L. hemionitideus was closely related to Lepisorus clathratus. The complete chloroplast genome of L. hemionitideus would provide very valuable molecular information for further inferring the relationships of the microsoroid ferns.
Leptochilus hemionitideus is terrestrial fern belonging to the subfamily Microsoroideae in Polypodiaceae (Zhang et al. Citation2013). Different from congeneric other species, it has orbicular to elongate sori on tertiary veins parallel to secondary veins (Zhang et al. Citation2013). Its fronds are not or only slightly dimorphic. The plant has rock habit, usually growing on stones in streams at an altitude of 700–2000 m (Zhang et al. Citation2013). Its main distribution areas are concentrated in China, Bhutan, India, Japan, Nepal and Thailand (Zhang et al. Citation2013). In China, L. hemionitideus is a traditional Chinese medical fern with clearing heat and detoxification (State Administration of Traditional Chinese Medicine ‘Chinese Materia Medica Committee’ Citation1999). In addition, whether Leptochilus were merged with Colysis occurs controversy due to lack of the obvious generic delimitation (Nooteboom Citation1997; Shi and Zhang Citation1999). Phylogenetic relationships of Leptochilus to some genera in Microsoroideae such as Microsorum are also needed to further explore (Christenhusz and Chase Citation2014; PPG I Citation2016). Therefore, sequencing complete chloroplast genome of L. hemionitideus will contribute to deal with these issues and lay solid foundations for further phylogenomic investigation.
We sampled fresh and young leaves of L. hemionitideus from South China Botanical Garden, Chinese Academy of Sciences (CAS; 23°11′3.56″N, 113°21′43.28″E). The voucher specimen was conserved in Herbarium of Sun Yat-sen University (SYS; voucher: SS Liu 20161014). After total genomic DNA extraction, we built up ∼300 bp genomic library and sequenced in Illumina Hiseq 2500 platform (Illumina Inc., San Diego, CA). Total 8,119,002 raw reads were retrieved and trimmed by Trimmomatic (Bolger et al. Citation2014). A subset of 6,801,257 trimmed reads was used for reconstructing the chloroplast genome by Velvet v1.2.07 (Zerbino and Birney Citation2008) with 33× coverage. The chloroplast genome was annotated using DOGMA (Wyman et al. Citation2004) and tRNAscan-SE (Schattner et al. Citation2005) programs with default settings and finally validated with BLAST searches and manually corrected for intron/exon boundaries. The complete chloroplast genome sequence of L. hemionitideus was aligned with 11 representative ferns including Marsilea crenata as outgroup using MAFFT v7.311 (Katoh and Standley Citation2013). A maximum likelihood (ML) phylogenetic tree was constructed using RAxML v.8.0 with 1000 bootstrap replicates (Stamatakis Citation2014).
We determined complete chloroplast genome of L. hemionitideus, which possesses a total length of 156,083 bp with 44.2% GC content (GenBank accession number: MH319943). The circular cp genome exhibits typical quadripartite with two inverted repeat regions (IRa and IRb) of 24,594 bp separated by a large single-copy (LSC) region of 81,403 bp and a small single-copy (SSC) region of 25,492 bp. It was predicted to contain 131 genes, including 87 protein-coding genes, 34 tRNA genes, eight rRNA genes and two pseudogenes (ndhB and rpoC1). Among them, 115 genes occur as a single copy, whereas 14 genes are duplicated in the IR regions. Fourteen genes contain one intron, especially, the gene ycf3, clpP, and rps12 have two introns. ML tree indicated that L. hemionitideus is closely related to Lepisorus clathratus (). The complete chloroplast genome of L. hemionitideus will provide very valuable molecular information for further inferring the relationships of the microsoroid ferns.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30:2114–2120.
- Christenhusz MJ, Chase MW. 2014. Trends and concepts in fern classification. Ann Bot. 113:571–594.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780.
- PPG I 2016. A community-derived classification for extant lycophytes and ferns. J Sytem Evol. 54:563–603.
- Shi L, Zhang XC. 1999. Taxonomic studies of the genus Colysis C. Presl (Polypodiaceae) from China and neighboring regions. Acta Phytotaxon Sin. 37:54–80.
- State Administration of Traditional Chinese Medicine ‘Chinese Materia Medica Committee’. 1999. Zhong Hua Ben Cao [Chinese Materia Medica], Vol. 4. Shanghai: Shanghai Science and Technology Publisher; p. 223–224. [Chinese]
- Schattner P, Brooks AN, Lowe TM. 2005. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33:W686–W689.
- Nooteboom H. 1997. The microsoroid ferns. Blumea. 42:261–395.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30:1312–1313.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20:3252–3255.
- Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829.
- Zhang XC, Lu SG, Lin YX, Qi XP, Moore S, Xing FW, Wang FG, Hovenkamp PH, Gilbert MG, Nooteboom HP, et al. 2013. Polypodiaceae. In: Wu ZY, Raven PH, Hong DY, eds., Flora of China. Vol. 2–3(Pteridophytes). Beijing: Science Press; p. 758–850.