Abstract
We obtained the mitogenome sequence of a Black Sea isolate of the kinetoplastid Bodo saltans. This sequence consists of two contigs totaling 24,925 bp and encodes ten protein-coding genes, one conserved ORF and one rRNA gene. Alignment of the Black Sea mitogenome with the limited sequence data currently available in public databases for another strain of B. saltans revealed significant genetic divergence between the two isolates. Maximum likelihood phylogenetic inference clearly resolved the Bodonidae from the Trypanosomatidae.
Kinetoplastida (phylum Euglenozoa) are protists whose distinctive feature is the presence of a kinetoplast, a network made of several circular DNA strands divided into minicircles and maxicircles. The maxicircles code for mitochondrial proteins, while the minicircles play a role in the maturation of the mRNA coded by the maxicircles (Lukeš et al. Citation2002). Kinetoplastids are commonly divided into parasitic and free-living (Opperdoes et al., Citation2016), the parasitic species being responsible of serious diseases like trypanosomiasis and leishmaniasis. Bodo saltans is a free-living species often found in polluted area, sewage water and eutrophic environment, where it feeds on bacteria (Mitchell et al. Citation1988; Doležel et al. Citation2000; Jackson et al. Citation2008). Only a 4040 bp fragment of the mitogenome from a freshwater B. saltans strain (Lake Constance) is currently available in GenBank (AF041263, Blom et al. Citation1998), and a genome project failed to reveal the putatively missing mitochondrial sequences (Jackson et al. Citation2008; ftp://ftp.sanger.ac.uk/pub/project/pathogens/Bodo/saltans/).
We sequenced total DNA from a B. saltans strain we isolated from Kazachia Bay in the Black Sea (44°34′19″N, 33°24′07″E). This strain was cultured in F/2 medium (20% salinity) enriched with yeast extract. Cells were ground in liquid nitrogen and DNA was extracted essentially as reported by Doyle and Doyle (Citation1990). An Illumina library of 300 bp DNA inserts was prepared and sequenced on the HiSeq 4000 platform by the Beijing Genomic Institute. A total of 32 million paired-end reads of 150 bp were obtained and assembled using SPAdes 3.12.0 (Bankevich et al. Citation2012) and a k-mer of 127.
Analysis of the 18S rRNA gene sequence retrieved from the assembled contigs (MH614643) revealed that it is identical to that of the B. saltans strain isolated from Gelendzhik, a site also located on the Northern part of the Black Sea (DQ207571, Schekenbach et al. 2006). Mitogenome sequences were recovered as two contigs totaling 24,925 bp in size. The 17,936 bp contig (MH614645) contains ten protein-coding genes (ND8, ND2, ND1, cox1 cox2, ND5, ND4, ND7, cox3, ATP6, and the conserved ORF MURF2) as well as a 540 bp fragment of the 12S rRNA gene (see De La Cruz et al. Citation1985; Horváth et al. Citation1990), while the other contig of 6989 bp (MH614644) contains only cob. The 9S rRNA and rps12 genes were not detected, but the latter could be encoded in a pan-edited G-rich region as reported for the Leishmania tarentolae kinetoplast maxicircle DNA (Maslov et al. Citation1992). The portion of the 17,936 bp contig spanning cox1 is co-linear with the previously reported sequence from the Lake Constance strain (AF041263). This gene shows 80.5% sequence identity between the two strains, with the Black Sea coding sequence starting with a TTG codon instead of ATG.
The total length of our mitogenome assembly falls in the same size range as those reported for complete kinetoplast maxicircles (e.g. DQ343646 in Westenberger et al. Citation2006). However, Blom et al (Citation1998) estimated that the B. saltans maxicircle could be as large as 70 kb, and recent long-read sequencing suggests that some kinetoplast maxicircles (CP022652, CM008275) could be up to 50 kb in size. It is likely that the sequences spanning the gaps between the two contigs we recovered contain abundant repeats, which prevented us from assembling the complete maxicircle using short reads. Future studies of the B. saltans mitogenome may thus benefit from third generation sequencing.
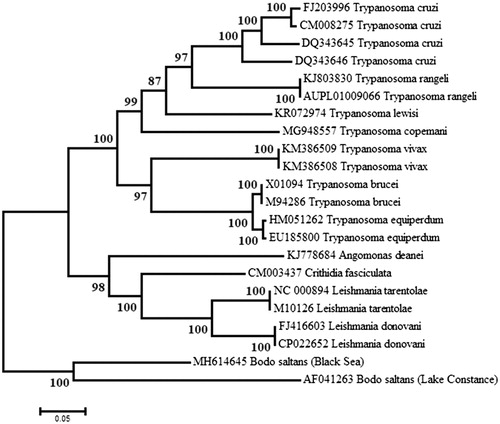
The sequences of a segment of about 3450 bp encoding the cox1 and cox2 genes of several kinetoplast maxicircles were aligned and a maximum likelihood tree was inferred using MEGA6 (Tamura et al. Citation2013). As expected, the two B. saltans strains were recovered in a highly supported clade that is distinct from the cluster containing the Trypanosomatidae ().
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bankevich A, Nurk S, Antipov D, Gurevich A, Dvorkin M, Kulikov AS, Lesin V, Nikolenko S, Pham S, Prjibelski A, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19:455–477.
- Blom D, de Haan A, van den Berg M, Sloof P, Jirku M, Lukes J, Benne R. 1998. RNA editing in the free-living bodonid Bodo saltans. Nucleic Acids Res. 26:1205–1213.
- De la Cruz VF, Simpson AM, Lake JA, Simpson L. 1985. Primary sequence and partial secondary structure of the 12S kinetoplast (mitochondrial) ribosomal RNA from Leishmania tarentolae: conservation of peptidyl-transferase structural elements. Nucleic Acids Res. 13:2337–2356.
- Doležel D, Jirkŭ M, Maslov DA, Lukeš J. 2000. Phylogeny of the bodonid flagellates (Kinetoplastida) based on small-subunit rRNA gene sequences. Int J Syst Evol Microbiol. 50:1943–1951.
- Doyle JJ, Doyle JL. 1990. Isolation of plant DNA from fresh tissue. Focus. 12:13–15.
- Horváth A, Maslov DA, Kolesnikov AA. 1990. The nucleotide sequence of the 12S ribosomal RNA gene from kinetoplast DNA of the protozoan Crithidia oncopelti. Nucleic Acids Res. 18:2811.
- Jackson AP, Quail MA, Berriman M. 2008. Insights into the genome sequence of a free-living Kinetoplastid: Bodo saltans (Kinetoplastida: Euglenozoa). BMC. 9:594.
- Lukeš J, Lys Guilbride D, Votýpka J, Zíková A, Benne R, Englund PT. 2002. Kinetoplast DNA network: evolution of an improbable structure. Eukaryot Cell. 1:495–502.
- Maslov DA, Sturm NR, Niner BM, Gruszynski ES, Peris M, Simpson L. 1992. An intergenic G-rich region in Leishmania tarentolae kinetoplast maxicircle DNA is a pan-edited cryptogene encoding ribosomal protein S12. Mol Cell Biol. 12:56–67.
- Mitchell GC, Baker JH, Sleigh MA. 1988. Feeding of a freshwater flagellate, Bodo saltans, on diverse bacteria. J Protozool. 35:219–222.
- Opperdoes FR, Butenko A, Flegontov P, Yurchenko V, Lukeš J. 2016. Comparative metabolism of free-living bodo saltans and parasitic trypanosomatids . J Eukaryot Microbiol. 63:657–678.
- Scheckenbach F, Wylezich C, Mylnikov AP, Weitere M, Arndt H. 2006. Molecular comparisons of freshwater and marine isolates of the same morphospecies of heterotrophic flagellates. J Appl Environ Microbiol. 72:6638–6643.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30:2725–2729.
- Westenberger SJ, Cerqueira GC, El-Sayed NM, Zingales B, Campbell DA, Sturm NR. 2006. Trypanosoma cruzi mitochondrial maxicircles display species- and strain-specific variation and a conserved element in the non-coding region. BMC. 7:60.