Abstract
The complete mitochondrial genome of the brownstripe red snapper, Lutjanus vitta was determined by MiSeq sequencing platform. The mitogenome of L. vitta (16,498 bp) encoded the typical 13 protein-coding genes, 22 tRNA genes, two rRNA genes, and two non-coding regions (origin of light strand replication; OL and D-Loop control region; CR). Phylogenetic analysis based on the full mitochondrial genome sequences showed that L. vitta is most closely related to L. russellii. The complete mitogenome sequence of L. vitta would provide the information of the genetic biodiversity in Lutjanus fishes, which would be further used for their scientific management in Indonesian waters.
The brownstripe red snapper, Lutjanus vitta is one of the economically important marine fish species, which is widely distributed in the western Pacific and Indian Ocean (Iwatsuki et al. Citation1993; Salini et al. Citation2006). Since L. vitta uses the coral reef as their spawning and feeding ground (Freitas et al. Citation2011), a high degree of genetic variations are expected considering its wide distribution along with coral reefs. The genetic information of L. vitta should be supplemented to manage scientifically its resource for the sustainable use.
Full mitochondrial genome sequence of L. vitta was determined by next-generation sequencing (NGS) platform. The specimen was collected from the coastal water in Pekalongan, Central Java, Indonesia (6°51'45″ S 109°4'24″ E) and stored at Universitas Airlangga, Indonesia. Species identification of the specimen was confirmed by both its morphological characteristics and DNA sequence identity in the COI region (GenBank Accession number: EU600101). Mitochondrial DNA was extracted by the mitochondrial DNA isolation kit (Abcam, UK) according to the manufacturer’s protocol. Purified mitochondrial DNA was further fragmented into smaller sizes (∼350 bp) by Covaris M220 Focused-Ultrasonicator (Covaris Inc., Woburn, MA, USA). A library for sequencing was constructed by TruSeq® RNA library preparation kit V2 (Illumina Inc., San Diego, CA, USA) and its quality and the quantity was analyzed by 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). DNA sequencing was performed by Illumina MiSeq sequencer (Illumina Inc., San Diego, CA, USA) (2 × 300 bp pair ends).
The complete mitochondrial genome of L. vitta (GenBank Number: MH675887) was 16,498 bp in length, which consisted of 13 protein-coding genes, 22 tRNAs, two ribosomal RNAs (12S and 16S), and two non-coding regions (origin of light strand replication; OL and D-Loop control region; CR). tRNA genes ranged from 69 bp to 76 bp in length. Eight tRNA genes were on L-strand while the other 14 tRNAs on H-strand formed the conserved three tRNA clusters (IQM, WANCY, and HSL) (Satoh et al. Citation2016). As a result of ARWEN (Laslett and Canbäck Citation2008), all the tRNAs were predicted to be folded into the typical clover-leaf secondary structures except for tRNA-Ser. Two non-coding regions, OL and CR, were located between tRNA-Asn and tRNA-Cys at WANCY cluster and between tRNA-Pro and tRNA-Phe, respectively. Besides COX1 gene, which was initiated by GTG, all other protein-coding genes begin with typical ATG start codons and incomplete stop codons were identified in ND2, COX2, COX3, ND3, ND4, and Cyt b genes.
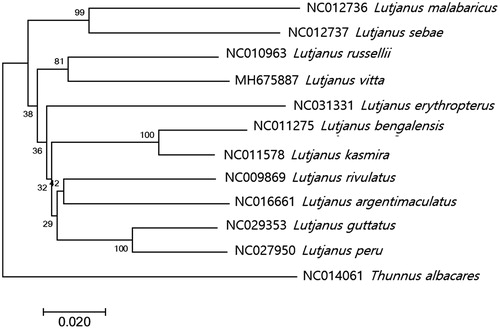
The phylogenetic tree of L. vitta was constructed with other 10 complete mitogenomes in Lutjanidae by MEGA7.0 program with minimum evolutionary (ME) algorithm (Kumar et al. Citation2016). Based on the full mitogenome sequences in the database, L. vitta was most closely related to L. russellii (NC010963) with 90% nucleotide sequence identity (). As the result with COX1 genes, L. vitta showed the highest identity to L. fulvus (93%, EU502667) followed by L. russellii (91%) suggesting L. fulvus may be the most closely related species to L. vitta among currently known Lutjanidae species in database.
Figure 1. Phylogenetic tree of Lutjanus vitta within Lutjanidae. Phylogenetic tree of Lutjanus vitta complete genome was constructed by MEGA7 software with Minimum Evolution (ME) algorithm with 1000 bootstrap replications. GenBank Accession numbers were shown followed by each scientific name. The sequence data for phylogenetic analyses used in this study were as follows: Lutjanus vitta (MH675887), Lutjanus russellii (NC010963), Lutjanus bengalensis (NC011275), Lutjanus kasmira (NC011578), Lutjanus rivulatus (NC009869), Lutjanus argentimaculatus (NC016661), Lutjanus peru (NC027950), Lutjanus guttatus (NC029353), Lutjanus erythropterus (NC031331), Lutjanus sebae (NC012736), Lutjanus malabaricus (NC012736), and furthermore Thunnus albacares (NC014061) as an outgroup.
Disclosure statement
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Freitas MO, De Moura RL, Francini-Filho RB, Minte-Vera CV. 2011. Spawning patterns of commercially important reef fish (Lutjanidae and Serranidae) in the tropical western South Atlantic. SCI MAR. 75:135–146.
- Iwatsuki Y, Akazaki M, Yoshino T. 1993. Validity of a lutjanid fish, Lutjanus ophuysenii (Bleeker) with a related species, L. vitta (Quoy et Gaimard). Jpn J Ichthyol. 40:47–59.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33:1870–1874.
- Laslett D, Canbäck B. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24:172–175.
- Salini J, Ovenden J, Street R, Pendrey R. 2006. Genetic population structure of red snappers (Lutjanus malabaricus Bloch & Schneider, 1801 and Lutjanus erythropterus Bloch, 1790) in central and eastern Indonesia and northern Australia. J Fish Biol. 68:217–234.
- Satoh TP, Miya M, Mabuchi K, Nishida M. 2016. Structure and variation of the mitochondrial genome of fishes. BMC Genomics. 17:719.
