Abstract
The endemic Madagascar banded commodore butterfly Precis andremiaja Boisduval, 1833 (Nymphalidae) inhabits forest margins and disturbed habitats. Genome skimming by Illumina sequencing allowed the assembly of a complete circular mitogenome of 15,239 bp from P. andremiaja consisting of 80.2% AT nucleotides, 22 tRNAs, 13 protein-coding genes, 2 rRNAs and a control region in the typical butterfly gene order. Precis andremiaja COX1 has a CGA start codon and COX1, COXII, NAD4 and NAD5 exhibit incomplete stop codons. Phylogenetic reconstruction places P. andremiaja as sister to Hypolimnas bolina within a monophyletic nymphalid tribe Junoniini, which is consistent with previous molecular phylogenetic hypotheses.
The butterfly genera Precis Hübner 1819 and Junonia Hübner 1819 share many morphological features and species have been moved back and forth between them since the nineteenth century (De Lesse Citation1952; Stecher Citation1969; Lalonde et al. Citation2018). Recent work suggests that Precis and Junonia are distinct and are not sister-taxa, with Precis being restricted to Africa, while Junonia is geographically widespread (Wahlberg et al. Citation2005). Most species in both genera have monomorphic wing colour patterns, but in correspondence with Charles Darwin in 1868, Henry Walter Bates identified the Madagascar banded commodore, P. andremiaja, as one of the only sexually dimorphic species (Stecher Citation1969). Precis andremiaja is endemic to Madagascar where it is found in disturbed areas and at forest edges (Lees et al. Citation2003). Here, we report the first complete mitochondrial genome sequence from genus Precis from P. andremiaja (specimen ID: Pand2014.1) that has been pinned, spread, and deposited in the Wallis Roughley Museum of Entomology, University of Manitoba (voucher JBWM0379997).
DNA was prepared (McCullagh and Marcus Citation2015) and sequenced by Illumina MiSeq (San Diego, California) (Peters and Marcus Citation2017). The mitogenome of P. andremiaja (Genbank MH917706) was assembled and annotated by Geneious 10.1.2 from 9,156,578 paired 300 bp reads using a Junonia lemonias (Lepidoptera: Nymphalidae) reference mitogenome (KP941756). The P. andremiaja nuclear rRNA repeat (Genbank MH917708) was assembled and annotated using Meroptera pravella (MF073208) (Living Prairie Mitogenomics Consortium Citation2017), Samia cynthia ricini (Saturniidae, AF463459) (Wang et al. Citation2003) and Papilio xuthus (Papilionidae, AB674749) (Futahashi et al. Citation2012) reference sequences.
The P. andremiaja circular 15,239 bp mitogenome assembly was composed of 13,790 paired reads with nucleotide composition: 39.8% A, 12% C, 7.6% G and 40.4% T. The gene composition and order in P. andremiaja matches that of other butterfly mitogenomes (McCullagh and Marcus Citation2015). Precis andremiaja mitochondrial protein coding genes use the start condons: ATG (COX2, ATP6, COX3, NAD4, NAD4L, CYTB, NAD1), ATT (NAD2, NAD3, NAD5, NAD6) ATC (ATP8) and CGA (COX1). The mitogenome contains four protein-coding genes (COX1, COX2, NAD4, NAD5) with single-nucleotide (T) stop codons completed by adding 3′ A residues post-transcriptionally. The locations and structures of tRNAs were determined using ARWEN v.1.2 (Laslett and Canback Citation2008). All have characteristic cloverleaf secondary structures except for trnS (AGN) where a loop replaces the dihydrouridine arm. The mitochondrial rRNAs and control region are typical of Lepidoptera (McCullagh and Marcus Citation2015).
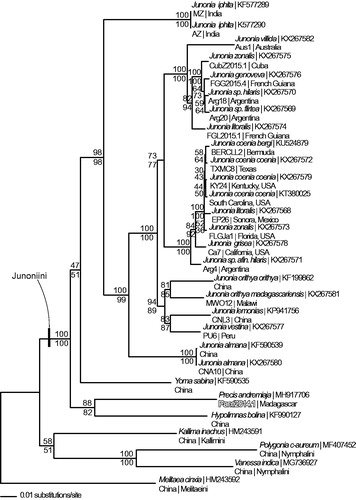
We reconstructed a phylogeny using complete mitogenomes from P. andremiaja, 24 representatives from tribe Junonini, and 4 outgroup species from other tribes within subfamily Nymphalinae (McCullagh and Marcus Citation2015; Peters and Marcus Citation2016; Peters and Marcus Citation2017; McCullagh and Marcus Citation2018). Mitogenome sequences were aligned in CLUSTAL Omega (Sievers et al. Citation2014) and analyzed by parsimony and maximum likelihood (model selected by jModeltest 2.1.7 (Darriba et al. Citation2012) and likelihood ratio test (Huelsenbeck and Rannala Citation1997)) in PAUP* 4.0b8/4.0d78 (Swofford Citation2002) (). Phylogenetic analysis places P. andremiaja as sister to Hypolimnas bolina within tribe Junoniini of subfamily Nymphalinae and is congruent with previous analyses (Wahlberg et al. Citation2005; Kodandaramaiah and Wahlberg Citation2007).
Figure 1. Phylogeny of tribe Junoniini: maximum likelihood phylogeny (GTR + G model, gamma = 0.1930, likelihood score 72678.06033) of Precis andremiaja, 24 additional mitogenomes from tribe Junoniini, and 4 outgroup species from other tribes in subfamily Nymphalinae based on 1 million random addition heuristic search replicates (with tree bisection and reconnection). One million maximum parsimony heuristic search replicates produced an identical tree topology (parsimony score 10,703 steps). Maximum likelihood bootstrap values and maximum parsimony bootstrap values (each from 1 million random fast addition search replicates) are above and below each node, respectively.
Acknowledgements
We thank Aleksandar Ilik and Debbie Tsuyuki (Children’s Hospital Research Institute of Manitoba Next Generation Sequencing Platform) for assistance with library preparation and sequencing.
Disclosure statement
The authors report no conflicts of interest, and are solely responsible for this paper.
Additional information
Funding
References
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 9:772.
- De Lesse H. 1952. Note sur les genres Precis Hb. et Junonia Hb. (Lep. Nymphalidae). Bull Soc Entomol France. 57:74–77.
- Futahashi R, Shirataki H, Narita T, Mita K, Fujiwara H. 2012. Comprehensive microarray-based analysis for stage-specific larval camouflage pattern-associated genes in the swallowtail butterfly, Papilio xuthus. BMC Biol. 10:46.
- Huelsenbeck JP, Rannala B. 1997. Phylogenetic methods come of age: Testing hypotheses in an evolutionary context. Science. 276:227–232.
- Kodandaramaiah U, Wahlberg N. 2007. Out-of-Africa origin and dispersal-mediated diversification of the butterfly genus Junonia (Nymphalidae: Nymphalinae). J Evol Biol. 20:2181–2191.
- Lalonde MML, McCullagh BS, Marcus JM. 2018. The taxonomy and population structure of the buckeye butterflies (Genus Junonia, Nymphalidae: Nymphalini) of Florida, USA. J Lepid Soc. 72:97–115.
- Laslett D, Canback B. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24:172–175.
- Lees DC, Kremen C, Raharitsimba T. 2003. Classification, diversity, and endemism of the butterflies (Papilionoidea and Herperioidea): A revised species checklist. In: Goodman SM, Benstead JP, editors. The natural history of Madagascar. Chicago and London: The University of Chicago Press; p. 762–792.
- Living Prairie Mitogenomics Consortium 2017. The complete mitochondrial genome of the lesser aspen webworm moth Meroptera pravella (Insecta: Lepidoptera: Pyralidae). Mitochondrial DNA B Resour. 2:344–346. doi:10.1080/23802359.2017.1334525
- McCullagh BS, Marcus JM. 2015. The complete mitochondrional genome of Lemon Pansy, Junonia lemonias (Lepidoptera: Nymphalidae: Nymphalinae). J Asia-Pacific Ent. 18:749–755.
- McCullagh BS, Marcus JM. 2018. When barcodes go bad: Exploring the limits of DNA barcoding with complete Junonia butterfly mitochondrial genomes Submitted to Molecular Phylogenetics and Evolution: Manuscript #MPE_2017_9.
- Peters MJ, Marcus JM. 2016. The complete mitochondrial genome of the Bermuda buckeye butterfly Junonia coenia bergi (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour. 1:739–741.
- Peters MJ, Marcus JM. 2017. Taxonomy as a hypothesis: testing the status of the Bermuda buckeye butterfly Junonia coenia bergi (Lepidoptera: Nymphalidae). Syst Entomol. 42:288–300.
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, et al. 2014. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 7:539.
- Stecher RM. 1969. Darwin-Bates Letters: Correspondence between two 19th-century travellers and naturalists. Ann Sci. 25:95–125.
- Swofford DL. 2002. PAUP*. Phylogenetic analysis using parsimony (*and Other Methods). Version 4. Sunderland, MA: Sinauer Associates.
- Wahlberg N, Brower AVZ, Nylin S. 2005. Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalinae (Lepidoptera: Nymphalidae). Biol J Linn Soc. 86:227–251.
- Wang SQ, Zhao MJ, Li TP. 2003. Complete sequence of the 10.3 kb silkworm Attacus ricini rDNA repeat, determination of the transcriptional initiation site and functional analysis of the intergenic spacer. DNA Sequence. 14:95–101.
