Abstract
Pteroceltis tatarinowii is an endangered Tertiary relict tree endemic to China with high ecological and economic value. Meanwhile, P. tatarinowii is the sole representative of the genus Pteroceltis Maxim., thus it is of great importance to the phylogenetic studies of Ulmaceae. Here, we first conducted the paired-end shotgun sequencing of P. tatarinowii on the Illumina HiSeq platform. Based on the resulting sequence, the complete chloroplast (cp) genome of P. tatarinowii was 158,635 bp long and displayed a typical quadripartite structure consisting of a pair of inverted repeats (IRs) with a length of 26,020 bp, separating by two single-copy regions (LSC, 87,627 bp and SSC, 18,968 bp). Besides, it encoded 119 genes, including 80 protein-coding genes, 29 transfer RNAs, eight ribosomal RNAs, and two pseudogenes. Moreover, a maximum likelihood (ML) phylogenetic analysis based on the 14 cp genomes revealed that P. tatarinowii was closely related to the genus Cannabis and the Ulmaceae species did not form a monophyletic clade.
Pteroceltis tatarinowii Maxim., the only representative of the genus Pteroceltis, is a temperate deciduous Tertiary relict tree distributed across the mainland of China (Fang Citation1996; Li et al. Citation2012) and it is renowned for the bark (phloem fiber) which used as the exclusive raw material of the traditional Chinese Xuan paper (Cao Citation1993). Moreover, P. tatarinowii is considered an ideal afforestation tree on bare limestone mountains because of its strong drought-resistant characteristic (Chen Citation1994; Li et al. Citation2015). In addition, P. tatarinowii has been categorized as a National Key Protected Species in China (Class III) (Fu Citation1992). In recent years, the population size of P. tatarinowii has decreased dramatically due to the over-exploitation for papermaking and habitat fragmentation, thus in urgent need of protection (Chai et al. Citation2010). Given the unprecedented rapid progress today in next-generation sequencing technologies, we can efficiently have access to the abundant genomic resources of our interested species (Yu et al. Citation2011). Here, we first reported the complete cp genome sequence of P. tatarinowii using the Illumina short-gun sequencing data and registered it into the GenBank with the accession number MH973587.
Total genomic DNA was extracted from the silica-dried leaves of one wild P. tatarinowii plant sampled from Langya Mountain (China; 32°17.1317'N, 118°17.0101'E) using a modified CTAB method (Yang et al. Citation2014) and the voucher specimen was deposited at the Herbarium of Zhejiang University (HZU). DNA library preparation and paired-end (2 × 125 bp) sequencing were performed on the Illumina HiSeq2500 platform at Beijing Genomics Institute (Shenzhen, China). Firstly, about 3.5 Gb of raw data were trimmed to remove low-quality reads (Q < 20, 0.01 probability error) and adapter sequences using the CLC-quality trim tool. Filtered reads were then assembled into contigs using CLC Genomics Workbench v8.5.1. All the high-quality contigs were aligned to the reference cp genome of Ulmus davidiana (KY244082; Zuo et al. Citation2017) via BLAST (http://blast.ncbi.nlm.nih.gov/) and the draft cp genome of P. tatarinowii was constructed by connecting the overlapping terminal sequences in GENEIOUS v11.0.4 (http://www.geneious.com/). Subsequently, clean reads were remapped to the draft cp genome and yielded the final cp genome sequence of P. tatarinowii. Finally, gene annotation was performed using the Dual Organellar GenoMe Annotator (Wyman et al. Citation2004).
The complete cp genome of P. tatarinowii was 158,635 bp long (with 36.3% GC content) and it displayed a typical quadripartite structure which consisted of a pair of inverted repeat regions (IRs with 26,020 bp) separated by two single-copy regions (LSC, 87,627 bp and SSC, 18,968 bp). Besides, there were 119 genes in P. tatarinowii cp genome including 80 protein-coding genes, 29 tRNA genes, eight rRNA genes and two pseudogenes, of which three protein-coding genes (rpl2, ndhA and ycf1) and one tRNA gene (trnL-TAA) contained a single intron, while three protein-coding genes (rpoC1, ycf3 and rps12) possessed two introns. The ψndhB and ψycf15 were identified as pseudogenes because of the partial duplication.
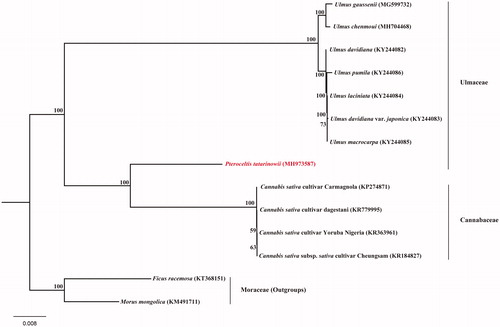
To further test the phylogentic placement of P. tatarinowii in Ulmaceae, we reconstructed the phylogeny of Ulmaceae based on the previously reported complete cp genomes of related taxa, employing the GTR + R + I model and 1000 bootstrap replicates under the maximum-likelihood (ML) inference in RAxML-HPC v.8.2.10 on the CIPRES cluster (Miller et al. Citation2010). Our phylogenetic tree showed the eight species of Ulmaceae did not form a paraphyletic clade and P. tatarinowii was closely related to the genus Cannabis (). These genomic resources and plastid phylogenomics here will largely enrich the genetic resources and enhance the conservation genetics of P. tatarinowii.
Disclosure statement
The authors report no conflicts of interest and are responsible for the content and writing of the paper.
Additional information
Funding
References
- Cao TS. 1993. Xuan paper of China. Beijing: China Light Industry Press; p. 20–34.
- Chai XY, Chen SL, Xu W. 2010. Using inter-simple sequence repeat markers to analyze the genetic structure of natural Pteroceltis tatarinowii populations and implications for species conservation. Plant Syst Evol. 285:65–73.
- Chen DG. 1994. Pteroceltis tatarinowii. Soil Water Conserve. Sinica. 11:36.
- Fang SZ. 1996. Cultivation of Pteroceltis tatarinowii and collecting and processing techniques for its barks. China Forest Sci Technol. 10:40–42.
- Fu LG. 1992. Chinese plant red book. Beijing: Science Press.
- Li XH, Shao JW, Lu C, Zhang XP, Qiu YX. 2012. Chloroplast phylogeography of a temperate tree Pteroceltis tatarinowii (Ulmaceae) in China. J Syst Evol. 50:325–333.
- Li XH, Zhang XP, Liu K, Liu HJ, Shao JW. 2015. Efficient development of polymorphic microsatellite loci for Pteroceltis tatarinowii (Ulmaceae). Genet Mol Res. 14:16444–16449.
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Gateway Computing Environments Workshop (GCE), New Orleans, LA, pp 1–8.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20:3252–3255.
- Yang JB, Li DZ, Li HT. 2014. Highly effective sequencing whole chloroplast genomes of angiosperms by nine novel universal primer pairs. Mol Ecol Resour. 14:1024–1031.
- Yu H, Tardivo L, Tam S, Weiner E, Gebreab F, Fan C, Svrzikapa N, Hirozane-Kishikawa T, Rietman E, Yang X, et al. 2011. Next-generation sequencing to generate interactome datasets. Nat Methods. 8:478–480.
- Zuo LH, Shang AQ, Zhang S, Yu XY, Ren YC, Yang MS, Wang JM. 2017. The first complete chloroplast genome sequences of Ulmus species by de novo sequencing: genome comparative and taxonomic position analysis. PLoS One. 12:e0171264.