Abstract
Peckia is one of the most important genera in the Sarcophagidae family of flesh flies. This genus is distributed in Brazil and Latin America, and its species can be used to estimate the Post Mortem Interval (PMI) in forensic investigations. In this communication, we present four mitochondrial genomes (mtDNA) from three Peckia species: P. australis, P. collusor, and P. resona. These mtDNA range from 15,116 bp to 15,234 bp in length and have 22 tRNA genes, 13 protein-coding genes (PCG), and two rRNAs distributed along both the strands. These data expand the knowledge about the Sarcophagidae genomes and present, for the first time, four complete mtDNA sequences of the Peckia genus. We show novel complete mtDNA sequences of flesh fly species of forensic importance. Our data expand the knowledge on the molecular database for the identification of these species, and is an important step towards increasing the databases and can help on the identification of new species, particularly in the forensic context.
Sarcophagidae is one of the most important families in forensic entomology. The genus Peckia has five subgenera and 67 species described (Pape Citation1996; Méndez and Pape Citation2003; Buenaventura and Pape Citation2013), and are mostly distributed in Latin America (Buenaventura and Pape Citation2015). Species of this genus are frequently used in forensic investigations (Moura et al. Citation1997; Carvalho et al. Citation2000). As observed in the genus Oxysarcodexia, the identification of Peckia species is based on male genitalia and has limitations, especially regarding the identification of larvae and female individuals (Buenaventura and Pape Citation2015), also of dandified specimens.
We collected species from different places in Brazil: P. australis (voucher NT2 – 23°18'27" S, 47°07'59" W), P. collusor (voucher 277 – 22°21'28" S 46°56'15" W), the two specimens of P. resona (voucher 264 – 23°10'13" S 46°53'53" W) and (voucher 402 – 31°45'59" S 52°19'46" W). Each specimen was identified following the taxonomic key (Buenaventura and Pape Citation2015) and preserved in 70% ethanol. The mtDNA was extracted from the thorax (Françoso et al. Citation2016), and the abdomen were deposited in the database of UNICAMP, according to the above-mentioned voucher codes. The mtDNA library was generated using the Nextera XT kit according to manufacturer’s instructions and sequenced using a paired-end strategy 2 × 250 on the MiSeq platform (Illumina, CA). The genomes were constructed by mapping the reads against the Sarcophagidae mitogenomes available on the NCBI database, followed by de novo assembly of the mapped reads using the CLC Genomics Workbench. The mitogenomes were annotated with the MITOS WebServer (Bernt et al. Citation2013) and manually verified using the NCBI database.
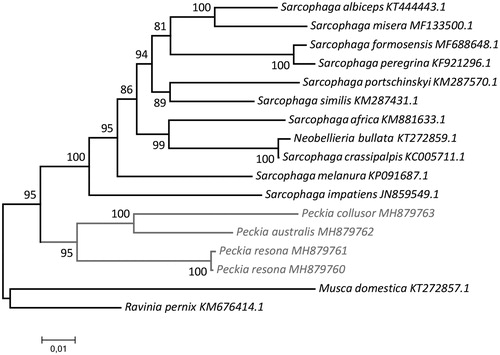
The complete mtDNA sequence of the P. australis (MH879762) was 15,205 bp long and had 23.3% of CG. Peckia collusor (MH879763) sequence was 15,234 bp long and showed 25.1% of CG. The mtDNA sequences of P. resona were 15,116 bp long with 23.5% of CG (voucher 264 – MH879761) and 15,122 bp long with 23.5% of GC (voucher 402 – MH879760). The two mitogenomes of P. resona differed in six additional bases located in the D-loop, probably due to the geographical separation of these populations by more than 1000 km. The annotation of these genomes revealed 13 protein-coding genes (PCGs), two rRNA genes, 22 tRNA genes, and a noncoding Control Region (D-loop) located between the 12S rRNA and tRNAIle. The PCG sequences commonly start with an ATT, ATA, or ATG codon (12 PCGs). COX1 showed an unusual start codon (CAA) for all the specimens. Five PCGs have the T– stop codon completed to TAA by posttranscriptional polyadenylation (Ojala et al. Citation1981). The light strand codifies eight of the 22 tRNAs, five PCGs, and the two rRNAs, while the remaining genes are encoded on the heavy strand. The phylogenetic tree () suggests that the Peckia clade is monophyletic.
Figure 1. Molecular phylogenetic inferences of Peckia mitogenomes. The phylogenetic analyses were made using the MEGA 7 software (Kumar et al.Citation2016) based on the maximum likelihood (ML) model with the nearest-neighbour-interchange heuristic method. The D-loop region was excluded from this analysis due to its high degree of variability (Gonder et al.Citation2007). The tree revealed that the Sarcophagidae species show a good degree of separation and structured branches, and suggests that the Peckia clade is monophyletic. The Peckia specimens are highlighted in grey. The mitogenomes for comparison were obtained from the NCBI database: Musca domestica (KT272857.1) – used as outgroup, Sarcophaga impatiens (JN859549.1), Sarcophaga melanura (KP091687.1), Neobellieria bullata (KT272859.1), Sarcophaga crassipalpis (KC005711.1), Sarcophaga peregrina (KF921296.1), Sarcophaga formosensis (MF688648.1), Sarcophaga africa (KM881633.1), Sarcophaga portschinskyi (KM287570.1), Sarcophaga similis (KM287431.1), Sarcophaga albiceps (KT444443.1), Sarcophaga misera (MF133500.1).
Acknowledgments
We thank the Universidade de Lisboa and the Universidade Federal de Minas Gerais (UFMG) for the support during the project execution.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69:313–319.
- Buenaventura E, Pape T. 2013. Revision of the New World genus Peckia Roniveau-Desvoidy (Diptera: Sarcophagidae). Zootaxa. 3622:1–87.
- Buenaventura E, Pape T. 2015. Phylogeny of the Peckia-Genus group: evolution of male genitalia in the major necrophagous guild of neotropical flesh flies (Diptera: Sarcophagidae). Org Divers Evol. 15:301–331.
- Carvalho L, Thyssen PJ, Linhares A, Palhares F. 2000. A checklist of Arthropods associated with pig carrion and human corpses in southeastern Brazil. Memorias Do Instituto Oswaldo Cruz. 95:135–138.
- Françoso E, Gomes F, Arias MC. 2016. A protocol for isolating insect mitochondrial genomes: a case study of NUMT in Melipona flavolineata (Hymenoptera: Apidae). Mitochondrial DNA. 27:24014. doi: 10.3109/19401736.2015.1028049
- Gonder MK, Mortensen HM, Reed FA, Sousa A, Tishkoff SA. 2007. Whole-mtDNA genome sequence analysis of ancient African lineages. Mol Biol Evol. 24:757–768. doi:10.1093/molbev/msl209.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7. 0 for bigger datasets. Mol Evol Genet Anal. 33:1870–1874.
- Méndez J, Pape T. 2003. Biology and immature stages of Peckia gulo (Fabricius, 1805) (Diptera:Sarcophagidae). Studia Dipterologica. 9:371–374.
- Moura MO, Carvalho CJB, Monteiro-Filho ELA. 1997. A preliminary analysis of insects of medico-legal importance in Curitiba, State of Paraná. Memorias Do Instituto Oswaldo Cruz. 92:269–274.
- Ojala D, Montoya J, Attardi G. 1981. TRNA punctuation model of RNA processing in human mitochondria. Nature. 290:470.
- Pape T. 1996. Catalogue of the Sarcophagidae of the world (Insecta: Diptera). Memoirs of Entomology International. 8:1–558.
