Abstract
Complete mitochondrial genome sequences were determined for five species of Australian freshwater fishes, representing a diverse range of ecologies and life histories. Mitogenomes were sequenced and annotated for Craterocephalus stramineus (Atherinidae); Hypseleotris klunzingeri (Eleotridae); Lovettia sealii (Galaxiidae); Leiopotherapon unicolor (Terapontidae) and Nematalosa erebi (Clupeidae). The five new mitogenomes each share the typical vertebrate mitochondrial arrangement of 13 protein coding genes, 22 tRNA genes, two rRNA genes and a control region. These sequences will be a useful resource for studies of evolutionary relationships and for management applications.
Introduction
The mitochondrial genome (mitogenome) is a small molecule (16–17,000 bp) that is present in most eukaryotic organisms. Vertebrate mitogenomes feature highly conserved gene composition and gene order (Satoh et al. Citation2016). Complete mitochondrial sequences are now available for >2,000 species of ray-finned fishes, and this growing resource plays a key role in evolutionary studies and in environmental management applications such as environmental DNA (eDNA) and metabarcoding (Sato et al. Citation2018). The Australian freshwater fish fauna, with just over 250 species, is relatively depauperate in species-level diversity, but highly endemic (Unmack Citation2001). Relatively few Australian freshwater fishes have mitogenome sequences available, although recent studies have demonstrated the utility of fully characterized mitogenomes for analyses of selection and population structure in high profile Australian freshwater fishes (e.g. Harrisson et al. Citation2016; Bishop et al. Citation2018). The aim of this study was to assemble and annotate the first complete mitochondrial genomes for several ecologically significant Australian freshwater fish species. The selected taxa have wide distributions across multiple drainage basins, and exhibit a diverse range of ecological characteristics. Tasmanian whitebait (Lovettia sealii, Galaxiidae) has a temperate distribution and a semi-anadromous life history (Schmidt et al. Citation2014). Strawman (Craterocephalus stramineus, Atherinidae) occurs in tropical drainages of northern Australia and is a habitat specialist (Mondol Citation2016). Spangled perch (Leiopotherapon unicolor, Terapontidae) is Australia’s most widely distributed freshwater fish, capable of extreme long-distance dispersal (Schmidt et al. Citation2018b). The western carp gudgeon (Hypseleotris klunzingeri, Eleotridae) is an abundant and widely distributed species which does not engage in interspecific hybridisation unlike other taxa in the carp gudgeon species complex (Schmidt et al. Citation2011). The distribution of bony bream (Nematolosa erebi) includes dryland rivers of central Australia and this species has broad temperature tolerance and high dispersal capability (Hughes and Hillyer Citation2006).
Methods
Low-coverage, whole-genome shotgun libraries were prepared for each species using randomly-sheared genomic DNA. A TruSeq (Illumina, San Diego, CA) library preparation kit was used with a targeted insert size of 500 bp (see Schmidt et al. Citation2018a). Paired-end sequencing was performed on the Illumina MiSeq Sequencer at Australian Genomics Research Facility (AGRF), using a 600 cycle MiSeq reagent kit v3. Novoplasty v2.6.5 was used to assemble the pair-end reads generated for each species into a single circular contig (Dierckxsens et al. Citation2017). Mitogenome annotation was achieved using MitoFish (Iwasaki et al. Citation2013). Tissue samples used for sequencing were derived from previous genetic studies and relevant specimen voucher codes and associated publication details are provided below.
Results and discussion
Lovettia sealii (GenBank accession: MK029347). DNA was obtained from specimen code 4021 collected from the Huon River, Tasmania (Lat. Long. −42.998483, 146.927883) (Schmidt et al. Citation2014). The seed sequence used to initiate assembly was Galaxias maculatus (NCBI RefSeq: NC_004594). A total of 3.8 × 106 reads were generated. Of these 4022 were incorporated into the new mitogenome assembly producing a 16,542 bp circular contig at 98× coverage.
Craterocephalus stramineus (GenBank accession: MK029350). DNA was obtained from specimen code DMC-CS8L-13 collected from the Daly River, Northern Territory (Lat. Long. −13.8092666, 131.341133) (Mondol Citation2016). The seed sequence used to initiate assembly was Craterocephalus eyresii (NCBI RefSeq: NC_035148). A total of 4.4 × 106 reads were generated. Of these 3,240 were incorporated into the new mitogenome assembly producing a 16,566 bp circular contig at 72x coverage.
Leiopotherapon unicolor (GenBank accession: MK024340). DNA was obtained from specimen code 11_04 collected from the Daly River, Northern Territory (Lat. Long. -14.3638, 131.557) (Schmidt et al. Citation2018a). The seed sequence used to initiate assembly was Scortum barcoo (NCBI RefSeq: NC_027171). A total of 3.4 × 106 reads were generated. Of these 996 were incorporated into the new mitogenome assembly producing a 16,527 bp circular contig at 20× coverage.
Hypseleotris klunzingeri (GenBank accession: MK029348). DNA was obtained from specimen code BR28 collected from the Balonne River at St George, Queensland (Lat. Long. −28.012917, 148.614753). The seed sequence used to initiate assembly was Hypseleotris sp. HAxHB (GenBank: KT716513) (Schmidt Citation2016). A total of 4.6 × 106 reads were generated. Of these 1710 were incorporated into the new mitogenome assembly producing a 16,508 bp circular contig at 37× coverage.
Nematalosa erebi (GenBank accession: MK029349). DNA was obtained from specimen code TM1 collected from the Bulloo River, Thargomindah, Queensland (Lat. Long. -28.017222, 143.785556) (Hughes and Hillyer Citation2006). The seed sequence used to initiate assembly was Nematalosa nasus (NCBI RefSeq: NC_023824). A total of 3.8 × 106 reads were generated. Of these 1,116 were incorporated into the new mitogenome assembly producing a 16,665 bp circular contig at 23x coverage.
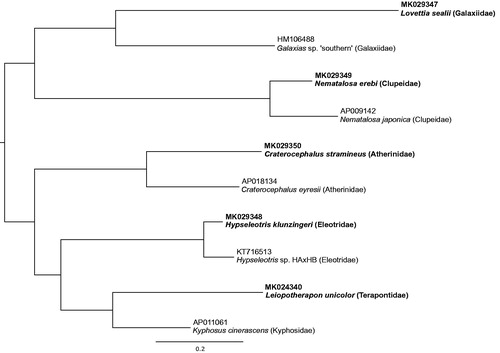
The five new mitogenomes all exhibit the typical gene order widely shared among vertebrates and the most common arrangement observed for fishes (Satoh et al. Citation2016). There was minor variation in the length of the control region among species: N. erebi, 1005 bp; L. sealii, 927 bp; L. unicolor, 792 bp; H. klunzingeri, 845 bp; C. stramineus, 883 bp. The new mitogenomes are unique additions to the NCBI nucleotide database as demonstrated by phylogenetic relationships () of the five new mitogenomes relative to the single closest match for each based on a BLASTn search (https://blast.ncbi.nlm.nih.gov).
Figure 1. Phylogenetic relationship of five new mitogenomes along with the single closest match for each one derived from BLASTn search. Tip labels include GenBank accession and species name. New mitogenomes highlighted in bold font. Alignment of mitogenomes (excluding 16S, 12S and control region) was performed using MAFFT v7.017 (Katoh et al.Citation2002). A maximum-likelihood phylogenetic analysis was performed on the final alignment of 11,500 bp with RAxML v8.2.11 using the GTR + GAMMA substitution model (Stamatakis Citation2006).
Acknowledgements
Samples were obtained from the tissue collection of the Molecular Ecology Laboratory, Griffith University. The authors thank Professor Jane Hughes for access to samples. Australian Genome Research Facility (AGRF) performed library preparation and sequencing. Funding was provided by a Griffith University, School of Environment, 2017 Research Grant.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bishop CR, Hughes JM, Schmidt DJ. 2018. Mitogenomic analysis of the Australian lungfish (Neoceratodus forsteri) reveals structuring of indigenous riverine populations and late Pleistocene movement between drainage basins. Conserv Gen. 19:587–597.
- Dierckxsens N, Mardulyn P, Smits G. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45:e18
- Harrisson K, Pavlova A, Gan HM, Lee YP, Austin CM, Sunnucks P. 2016. Pleistocene divergence across a mountain range and the influence of selection on mitogenome evolution in threatened Australian freshwater cod species. Heredity. 116:506–515.
- Hughes JM, Hillyer MJ. 2006. Mitochondrial DNA and allozymes reveal high dispersal abilities and historical movement across drainage boundaries in two species of freshwater fishes from inland rivers in Queensland, Australia. J Fish Biol. 68:270–291.
- Iwasaki W, Fukunaga T, Isagozawa R, Yamada K, Maeda Y, Satoh TP, Sado T, Mabuchi K, Takeshima H, Miya M, et al. 2013. MitoFish and MitoAnnotator: a mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol Biol Evol. 30:2531–2540.
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30:3059–3066.
- Mondol RKM. 2016. Population connectivity of two Australian freshwater fishes in a large tropical dentritic river network PhD Thesis, Griffith University: Griffith University.
- Sato Y, Miya M, Fukunaga T, Sado T, Iwasaki W. 2018. MitoFish and MiFish Pipeline: a mitochondrial genome database of fish with an analysis pipeline for environmental DNA Metabarcoding. Mol Biol Evol. 35:1553–1555.
- Satoh TP, Miya M, Mabuchi K, Nishida M. 2016. Structure and variation of the mitochondrial genome of fishes. Bmc Genomics. 17:719.
- Schmidt DJ. 2016. The complete mitogenome of an Australian carp gudgeon, hybridogenetic biotype HAHB (Hypseleotris: Eleotridae). Mitochondrial DNA A. 27:4582–4583.
- Schmidt DJ, Bond NR, Adams M, Hughes JM. 2011. Cytonuclear evidence for hybridogenetic reproduction in natural populations of the Australian carp gudgeon (Hypseleotris: Eleotridae). Mol Ecol. 20:3367–3380.
- Schmidt DJ, Crook DA, Macdonald JI, Huey JA, Zampatti BP, Chilcott S, Raadik TA, Hughes JM. 2014. Migration history and stock structure of two putatively diadromous teleost fishes, as determined by genetic and otolith chemistry analyses. Freshwater Sci. 33:193–206.
- Schmidt DJ, Espinoza T, Real K, Dunlop A, Kennard M, Hughes JM. 2018a. Improved genetic markers for monitoring recruitment dynamics in the endangered Mary River cod (Maccullochella mariensis). J Appl Ichthyol. 34:633–637.
- Schmidt DJ, Huey JA, Hughes JM. 2018b. Genome-wide SNPs identify limits to connectivity in the extreme freshwater disperser, Spangled Perch Leiopotherapon unicolor (Terapontidae). J Hered. 109:320–325.
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 22:2688–2690.
- Unmack PJ. 2001. Biogeography of Australian freshwater fishes. J Biogeogr. 28:1053–1089.
