Abstract
The full mitogenome of Bothrocara hollandi was determined by high-throughput sequencing (HTS) platform. The genome was 16,732 bp in length, which included 13 protein-coding genes, 22 tRNAs, two ribosomal RNAs (12S and 16S), and a control region (D-Loop). The overall composition of G + C in the mitogenome was 45.90%, which was lower than A + T contents (54.10%). Except for COX1 (GTG), the other twelve protein-coding genes begin with typical start codon, ATG. The incomplete stop codon (TA − or T–) was identified in five genes, including COXI, COXII, COXIII, ND3, and ND4. The phylogenetic analysis with the currently reported mitogenome sequences showed that B. hollandi was most closely related to Lycodes tanakae, suggesting that additional mitogenome sequences in genus Bothrocara are required for its clear evolutional relationship.
The porous-head eelpout, Bothrocara hollandi is one of dominant deep-sea demersal fish resources in East/Japan Sea (Kojima et al. Citation2001), which inhabits between 150 and 1980 m in depth (Okiyama Citation2004). This species is paid attention as the economically important deep-sea fish resources in East/Japan Sea and its scientific management is currently required. Although several genetic population studies have been made with partial DNA sequences (Kojima et al. Citation2001; Kojima et al. Citation2011), the complete mitochondrial genome sequence of B. hollandi is still not yet reported.
The sample of B. hollandi was collected from East/Japan Sea (36°36′03″N, 129°56′08″E) as the part of ‘deep-sea resource survey’ project of Dokdo Research Center in National Institute of Fisheries Sciences (NIFS), Republic of Korea. The species of a specimen was identified by both morphological and molecular analysis, which showed 100% identity to the sequence in the database (GenBank number: KC748099) in COI region. The specimen was stored at the Department of Marine Biology in Pukyong National University. The full mitochondrial genome sequence of B. hollandi was determined by Illumina Miseq platform with the extracted mitochondrial DNAs using a commercial purification kit (Abcam, Cambridge, UK). A library for MiSeq sequencing (2 × 300 bp pair ends) was constructed by TruSeq® RNA library preparation kit version 2 (Illumina, San Diego, CA) with the fragmented mitochondrial DNAs (∼350 bp) by Covaris M220 Focused-Ultrasonicator (Covaris Inc., Woburn, MA). The mitochondrial genome was constructed by the multiple assembly of short fragmental reads using Geneious software version11.0.2 (Kearse et al. Citation2012).
The complete mitochondrial genome of B. hollandi (GenBank Number: MK069496) was 16,732 bp in length, which consisted of 13 protein-coding genes, 22 tRNAs, 2 ribosomal RNAs (12S and 16S) as well as a non-coding control region (D-Loop). The overall composition of G + C in mitogenome was 45.90%, which was lower than A + T contents (54.10%). Twenty-nine genes were located on the heavy (H) strand, and remaining eight genes were located on the light (L) strand. Twelve protein-coding genes were started with common ATG except for COX1 (GTG). The incomplete stop codon (TA − and T–) was identified in five protein-coding genes including COXI, COXII, COXIII, ND3, and ND4. As the result of ARWEN (Laslett and Canbäck Citation2008), 21 tRNA genes were able to be folded into the typical clover secondary structures, except for tRNA-Ser(AGC).
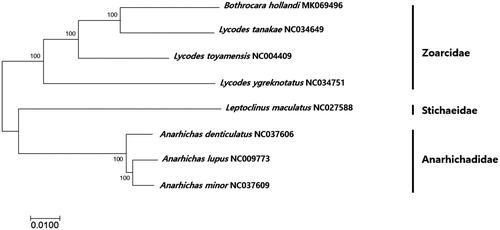
The phylogenetic tree of B. hollandi was constructed with the currently deposited mitogenomes in Zoarcidae, Stichaeidae and Anarhichadidae using MEGA version 7.0 software with minimum evolution (ME) algorithm (Kumar et al. Citation2016). Based on the phylogenetic analysis, B. hollandi was the most closely related to a fish in other genus, Lycodes tanakae (Kim et al. Citation2016) with 93% nucleotide sequence identity (). This is the first report about the mitogenome sequence in genus Bothrocara and more mitogenome sequences in this genus should be supplemented to obtain better insight about the phylogenetic and evolutional relationship of species in the family Zoarcidae.
Disclosure statement
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Funding
References
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28:1647–1649.
- Kim YK, Song YS, Kim IH, Kang CB, Kim WB, Kim SY. 2016. Complete mitochondrial genome of Lycodes tanakae (Perciformes: Zoarcidae). Mitochondrial DNA Part B. 1:947–948.
- Kojima S, Maeda R, Sakuma K, Kokubu Y, Hagihara S, Itoh M. 2011. Genetic characterization of the Northwestern Pacific population of a deep-sea demersal fish, Bothrocara hollandi. Plankton Benthos Res. 6:108–114.
- Kojima S, Segawa R, Hayashi I, Okiyama M. 2001. Phylogeography of a deep-sea demersal fish, Bothrocara hollandi, in the Japan Sea. Mar Ecol Prog Ser. 217:135–143.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33:1870–1874.
- Laslett D, Canbäck B. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24:172–175.
- Okiyama M. 2004. Deepest demersal fish community in the Sea of Japan: a review. Contr Biol Lab Kyoto Univ. 29:409–429.