Abstract
This study was the first report about complete chloroplast genome of Gymnocoronis spilanthoides (Asteraceae, Eupatorieae), a critically invasive plant. The circular whole cp genome of G. spilanthoides was 150,799 bp in length, contained a large single-copy (LSC) region of 82,514 bp and a small single-copy (SSC) region of 18,471 bp. These two regions were separated by a pair of inverted repeat regions (IRa and IRb), each of them 24,907 bp in length. A total of 135 functional genes were encoded, consisted of 89 protein-coding genes, 38 tRNA genes, and eight rRNA genes. The overall GC content of the chloroplast genome sequence was 37.5%, and the GC contents of the LSC, SSC, and IR regions were 35.6, 30.9, and 37.0%, respectively. The phylogenetic analysis by maximum likelihood showed that the G. spilanthoides was clustered into clade of Mikania and Ageratina by strong support values, and thus was closely related to members of Heliantheae and Inuleae. These results will be useful for the future studies of the naturalized and potentially invasive aquatic alien in the worldwide.
Gymnocoronis spilanthoides (D. Don ex Hook. Arn.) DC. (Asteraceae, Eupatorieae) is a neotropical species, native to Peru, N Argentina, Bolivia, Paraguay, Uruguay, and Brazil (Hind and Robinson Citation2007; Chen et al. Citation2011), and it is also the critically invasive plant species, recently naturalized in Asia (Suyama Citation2001; Gao and Liu Citation2007; Lu et al. Citation2018), Australia, Europe, and New Zealand (Webb et al. Citation1995; Ardenghi et al. Citation2016). Genetic knowledge of G. spilanthoides would provide information for protection of this wild germplasm resource. Here, we obtained the complete plastome of G. spilanthoides using Illumina sequencing technology. The complete plastome reported here will contribute to the further studies on the phylogenetic analysis and prevention and controlling genetics of G. spilanthoides.
Fresh leaves of G. spilanthoides were collected from Sichuan Province, China and deposited in Herbarium, Sichuan Normal University, SCNU (specimen no.: Z.X. Fu 3035). High quality total genomic DNA was extracted from ca.6 cm2 sections of the silica-dried leaf using improved Tiangen Plant Genomic DNA Kits, add the 4 μl RNAseA and 20 μl Proteinase K after incubated (65 °C). Total DNA was directly constructed short-insert of 150 bp in length libraries and sequenced on the Illumina Genome Analyzer (Hiseq 2000) based the manufacturer’s protocol (Illumina, San Diego, CA, USA) using ORI-GENE, Beijing. Generally, more than 6 Gb of data was obtained for complete cp genome of G. spilanthoides. De novo assembled in CLC Genomic Workbench v11 (CLC Bio, Aarhus, Denmark) and consensus sequence in Geneious R11.1.5 (Biomatters Ltd., Auckland, New Zealand) with referenced chloroplast genome sequence of Mikania micrantha H.B.K (Accession: KX154571). The chloroplast genome were annotated using a web-based annotation program GeSeq (https://chlorobox.mpimp-golm.mpg.de/geseq.html) and editing by manual and imagining with OGDraw v1.2 (Lohse et al. Citation2013).
The complete chloroplast genome of G. spilanthoides was 150,799 bp in length and a typical circular structure, which comprising a pair of inverted repeat (IR) of 24,907 bp divided by a large single copy (LSC) region of 82,514 bp and a small single copy (SSC) region of 18,471 bp. The general G + C content was 37.5% in the whole sequence, whereas corresponding values of 35.6%, 30.9%, and 37.0% in the LSC, SSC, and IR regions. The whole genome contained 135 genes, including 89 protein-coding genes, eight ribosomal RNA genes, and 38 tRNA genes, nevertheless, 114 unique genes, 20 genes duplicated in the IRs. In addition, among the annotated chloroplast genomic sequence, 15 genes possessed only single intron, two genes (ycf3 and clpP) possessed two introns.
To identify the phylogenetic position of G. spilanthoides, we used a total of 20 additional complete cp genomes of the subfamily Asteroideae and outgroup taxa to clarify the phylogenetic position of G. spilanthoides (). All of the cp genome sequences were aligned in MAFFT (Katoh and Standley Citation2013). A maximum likelihood analysis based on the GTRGAMMA model was performed with RaxML v7.2.8 on the CIPRES (Stamatakis et al. Citation2008; Miller et al. Citation2010) using 1000 bootstrap replicates. The phylogenetic analysis of the cp genome dataset recovers the similar clades as in previous phylogenetic work (Panero and Funk Citation2008; Panero et al. Citation2014; Fu et al. Citation2016). ML result with 100% bootstrap showed that G. spilanthoides has a close sister relationship with Ageratina adenophora and Mikania micrantha ().
Figure 1. The best Maximum likelihood (ML) phylogram inferred from 21 chloroplast genomes in Asteraceae (bootstrap value are indicated on the branches). The position of Gymnocoronis spilanthoides is shown in a box.
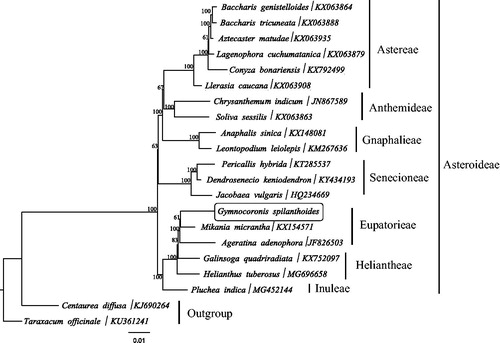
The complete cp genome sequence of G. spilanthoides will be the valuable resource for future studies on taxonomy and phylogeny of family Asteraceae and provides useful molecular data for further phylogenetic and evolutionary analysis.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Ardenghi NMG, Barcheri G, Ballerini C, Cauzzi P, guzzon F. 2016. Gymnocoronis spilanthoides (Asteraceae, Eupatorieae), a new naturalized and potentially invasive aquatic alien in S Europe. Willdenowia. 46:265–273.
- Chen YL, Kawahara T, Hind DJN. 2011. Eupatorieae. In: Wu CY, Raven PH, Hong DY, editors. Flora of China, Vol. 20-21. Beijing: Science Press & St. Louis: Missouri Botanical Garden Press; p. 879–891.
- Fu ZX, Jiao BH, Nie B, Zhang GJ, Gao TG. 2016. A comprehensive generic-level phylogeny of the sunflower family: Implications for the systematics of Chinese Asteraceae. J Syst Evol. 54:416–437.
- Gao TG, Liu Y. 2007. Gymnocoronis a new naturalized genus of the tribe Eupatorieae, Asteraceae in China. Acta Phytotax Sin. 45:329–332.
- Hind DJN, Robinson H. 2007. Gymnocoronis. In: Kadereirt J, Jeffrey C, editors. The families and genera of vascular plants, Vol. 8. Berlin and Heidelberg: Springer; p. 519–520.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780.
- Lohse M, Drechsel O, Kahlau S, Bock R. 2013. Organellar Genome DRAW—a suite of tools for generating physical maps of plastid and mitochondrial genomes and vsualizing expression data sets. Nucl Acids Res. 41:575–581.
- Lu X, Yang XC, Peng SM, Gao TG, Hu GX. 2018. Gymnocoronis spilianthoides, a new invasive species (Eupatotieae, Asteraceae) in Sichuan. China Plant Quar. 32:73–75.
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees. Proceedings of the gateway computing environments workshop (GCE). Nov 14–14; New Orleans (LA): IEEE; p. 1–8.
- Panero JL, Funk VA. 2008. The value of sampling anomalous taxa in phylogenetic studies: major clades of the Asteraceae revealed. Mol Phylogenet Evol. 47:757–782.
- Panero JL, Susana EF, Espinar LA, Crozier BS, Barboza GE, Cantero JJ. 2014. Resolution of deep nodes yields an improved backbone phylogeny and a new basal lineage to study early evolution of Asteraceae. Mol Phylogenet Evol. 80:43–53.
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol. 57:758–771.
- Suyama C. 2001. A new naturalized plant, Gymnocoronis spilanthoides. J Phytogeogr Taxon. 49:183–184.
- Webb CJ, Sykes WR, Garnock-Jones PJ, Brownsey PJ. 1995. Checklist of dicotyledons, gymnosperms, and pteridophytes naturalised or casual in New Zealand: additional records 1988–1993. New Zeal J Bot. 33:151–182.
