Abstract
Biomphalaria straminea is an invasive species in China and is one of the intermediate hosts of Schistosoma mansoni which results in the spread of schistosomiasis. Herein, we firstly report the complete mitochondrial genome of B. straminea. The full length of the mitochondrial genome is 13,652 bp. It consists of 37 typical animal mitochondrial genes, comprising 13 protein-coding genes, 22 transfer RNA genes, and two ribosomal RNA genes. The contents of each base in the complete mitochondrial genome are 33.28% A, 42.01% T, 10.85% C, and 13.87% G, with a high A + T content of 75.29%. Phylogenetic analysis showed that the relationship of B. straminea and Biomphalaria tenagophila was more closed.
Biological invasion phenomenon is one of the most important problems and occurs widely in the world (Mooney and Hobbs Citation2000; Ricciardi Citation2007). Biomphalaria straminea (Dunker 1848) is mainly distributed in Brazil and then spread to the several islands of the Lesser Antilles, Caribbean, and Costa Rica (Pointier Citation1993). Biomphalaria straminea was discovered in 1973 by Meier Brook in Hong Kong, China, it was assumed that this snail was introduced to China along with imported plants or fish from South America (Meier-Brook Citation1974). And then B. straminea was discovered in Shenzhen City (Wang and Zhang Citation1985). Currently, in addition to Hongkong, Shenzhen city, it was also found in Dongguan City, Huizhou city and other places (Gao et al. Citation2013). This invasive species has a tendency of expansion in the further in China. It has been predicted that B. straminea will spread to Pearl River Delta of Guangdong, southern parts of Guangxi and Fujian provinces, and the North of Taiwan (Habib et al. Citation2016). However, in recent years, it is a sharp increase in the number of Africa-aid projects, and more cases infected with schistosomiasis have been detected when the workers returned to China from Africa (Zhou et al. Citation2011). The presence of people who infected with schistosomiasis and high-density distribution of B. straminea in some areas of China, it implies that the epidemic or transmission of Schistosomiasis mansoni may have happened in China.
Here, we sequenced the complete mitochondrial genome of B. straminea. At the same time, we also analyzed phylogenetic relationship based on the mitochondrial protein sequences of 13 mollusc species. These results will provide useful information and scientific basis for prediction of the dispersal of B. straminea and preventing of the spread of schistosomiasis.
The samples were collected from Dasha River (22° 34′ 76″ N; 113° 57′ 39″ E), Shenzhen city, in 2016, and transported to the laboratory for storage in 90% ethanol. Tissues of individual specimens were preserved in 95% ethanol and stored at −20 °C until DNA extraction. The genomic DNA was further extracted from foot tissue of snails examined above using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) and kept at −20 °C. Concentration and quality of extracted DNA were estimated using a Nanodrop 2000 (Thermo Scientific) and agarose gel electrophoresis. Mitochondrial genome data were generated by Beijing GGT Co., Ltd Company.
The length of complete mitochondrial genome of B. straminea was 13,652 bp (GenBank: MF480756) and included 13 protein-coding genes (PCGs), 22 tRNA genes, and two rRNA genes. The total base composition of the entire genome was as follows: A 33.28%, T 42.01%, C 10.85%, and G 13.87%, with a high A + T content of 75.29%. The heavy DNA strand (H-strand) contains most of the genes: nine protein-coding genes, rrnL gene, and 14 tRNA genes. Four protein-coding genes, rrnS gene, and eight tRNA genes are located on the L-strand.
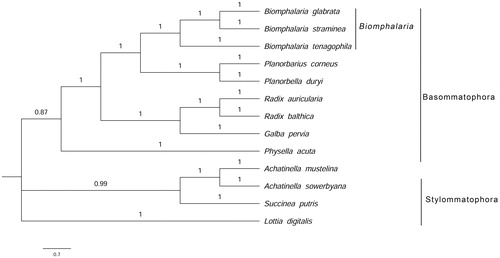
Phylogenetic analysis based on the 12 protein-coding genes showed that the members of the family Planorbidae are clustered together. Compared with Biomphalaria tenagophila, the relationship of B. straminea and Biomphalaria glabrata is more closed ().
Figure 1. Phylogenetic analysis of mitochondrial protein sequences of 13 mollusc species using Bayesian inference method. (GenBank accession numbers, Achatinella mustelina: NC030190; Achatinella sowerbyana: KX356680; Biomphalaria glabrata: NC005439; Biomphalaria straminea: MF480756; Biomphalaria tenagophila: NC010220; Galba pervia: NC018536; Lottia digitalis: NC007782; Physella acuta: NC023253; Planorbarius corneus: NC026708; Planorbella duryi: KY514384; Radix auricularia: NC026538; Radix balthica: NC026539; Succinea putris: NC016190).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Gao ST, Li XH, Huang SY, Xie X, Mei SJ, Ruan CW, Huang DN. 2013. Primary investigation of distribution and ecological environment of Biomphalaria straminea in Dasha and Guanlan Rivers in Shenzhen areas. China Tropica Med. 13:313–331.
- Habib MR, Guo YH, Lv S, Gu WB, Li XH, Zhou XN. 2016. Predicting the spatial distribution of Biomphalaria straminea, a potential intermediate host for Shistosoma mansoni, in China. Geospat Health. 11:375–383.
- Meier-Brook C. 1974. A snail intermediate host of Schistosoma mansoni introduced into Hong Kong. Bull World Health Organ. 51:661.
- Mooney HA, Hobbs RG. 2000. Invasive species in a changing world. Covelo (CA): Island Press.
- Pointier JP. 1993. The introduction of Melanoides tuberculata (Mollusca: Thiaridae) to the island of Saint Lucia (West Indies) and its role in the decline of Biomphalaria glabrata, the snail intermediate host of Schistosoma mansoni. Acta Tropica. 54:13–18.
- Ricciardi A. 2007. Are Modern Biological Invasions an Unprecedented Form of Global Change? Conserv. Biol. 21:329–336.
- Wang YX, Zhang WZ. 1985. The intermediate hosts of Schistosoma mansoni—Biomphalaria straminea. Chinese J Zool. 6:18–20.
- Zhou Y, Qi ZQ, Feng ML, Wang F, Li W, Li SG, Xu CB, Gu JC. 2011. Clinical analysis of imported Schistosoma mansoni infections: a report of two casae and review of the literature. China Tropica Med. 11:250–252.
