Abstract
Pseudostellaria palibiniana which belongs to subseries Verticilatae is one of the species in Pseudostellaria palibiniana species complex (PPSC). To uncover intraspecies variation of P. palibiniana, we presented its second complete chloroplast genome which is 149,639 bp long and has four subregions: 81,286 bp of large single copy and 16,977 bp of small single copy regions are separated by 25,688 bp of inverted repeat regions including 126 genes (81 protein-coding genes, 8 rRNAs, and 37 tRNAs). The overall GC content is 36.5% and those in the LSC, SSC, and IR regions are 34.3%, 29.4%, and 42.4%, respectively. Eighty-four single nucleotide polymorphisms and 125 insertions and deletions are identified between two individuals of P. palibiniana. Phylogenetic tree presents that P. palibiniana isolated from the same place of Pseudostellaria longipedicellata is clustered with another P. palibiniana, showing that PPSC can be solved using complete chloroplast genomes.
Pseudostellaria palibiniana (Takeda) Ohwi belongs to subseries Verticilatae of which key morphological characteristics is pseudo-whorl (Ohwi Citation1937): leaves are attached at almost equal levels and separated by single fully elongated internodes (Kwiatkowska Citation1999). It is distributed only in Korean peninsula and Honshu in Japan (Ōi et al. Citation1965; Park Citation2007). In subseries Verticilatae, at least three Pseudostellaria species, which are endemic in Korea (Ohwi Citation1937; Lee et al. Citation2012) have been identified. Based on the molecular phylogeny, these species are not resolved clearly, forming Pseudostellaria palibiniana species complex (PPSC; Kim et al., in preparation). To resolve phylogenetic relations in PPSC by high resolution of marker sequences (Zhang et al. Citation2017), we sequenced a second complete chloroplast genome sequence of P. palibinina isolated from Mt. Taebaek, Gangwon Province, Korea (Voucher in InfoBoss Cyber Herbarium (IN); Y. Kim, IB-00577), which is different from the first chloroplast chloroplast genome.
Total DNA was extracted from fresh leaves using a DNeasy Plant Mini Kit (QIAGEN, Hilden, Germany). Raw sequences were obtained by HiSeq2000 (Macrogen Inc., Seoul, Korea), de novo assembly was done by Velvet 1.2.10 (Zerbino and Birney Citation2008), and confirmed by BWA 0.7.17 and SAMtools 1.9 (Li et al. Citation2009; Li Citation2013). Geneious R11 11.0.5 (Biomatters Ltd., Auckland, New Zealand) was used for chloroplast genome annotation based on first P. palibiniana chloroplast genome (MK120981; Kim et al., under revision).
The chloroplast genome of P. palibiniana (Genbank accession is MK309611) is 149,639 bp long and has four subregions: 81,286 bp of large single copy (LSC) and 16,977 bp of small single copy (SSC) regions are separated by 25,688 bp of inverted repeat (IR). It contained 126 genes (81 protein-coding genes, 8 rRNAs, and 37 tRNAs); 15 genes (four protein-coding genes, four rRNAs, and seven tRNAs) are duplicated in IR regions. The overall GC content is 36.5% and those in the LSC, SSC, and IR regions are 34.3%, 29.4%, and 42.4%, respectively.
Based on the pairwise alignment of two P. palibiniana chloroplast genomes, 84 single nucleotide polymorphisms (SNPs) and 125 insertions and deletions (INDELs) are identified. Only one SNP (2.4%) and 21 INDELs (33.6%) are in the IR region. Fourteen of 81 protein-coding genes have 29 SNPs (34.5%) and 12 INDELs (9.6%). Twelve INDELs found in ycf4 do not cause frameshift.
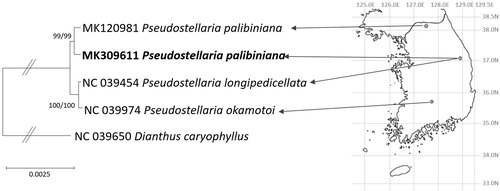
Four complete Pseudostellaria chloroplast genomes (Kim et al. Citation2018, Citation2019) and one Dianthus chloroplast genome as an outgroup were used for constructing maximum likelihood (1,000 repeats) and neighbor joining (10,000 repeats) bootstrapped phylogenetic trees using MEGA X (Kumar et al. Citation2018). Alignment was conducted by MAFFT 7.388 (Katoh and Standley Citation2013). Phylogenetic trees show that two P. palibiniana chloroplast genomes are monophyletic; while the rest of the species in Pseudostellaria form another clade. Considering the same location of second P. palibiniana and P. longipedicellata (Kim et al. Citation2018), two Pseudostellaria species are clearly distinguished with complete chloroplast genome, indicating that complete chloroplast genome may have enough resolution to resolve PPSC (Kim et al., in preparation; ). With more complete Pseudostellaria chloroplast genomes, the evolutionary history of species in PPSC can be resolved.
Figure 1. Maximum likelihood and neighbor joining phylogenetic trees of Caryophyllaceae based on five complete chloroplast genomes: Pseudostellaria palibiniana, (MK309611 in this study and MK120981), Pseudostellaria okamotoi (MH879018), Pseudostellaria longipedicellata (MH373593), and Dianthus caryophyllus (NC_039650). The numbers above branches indicate bootstrap support values of neighbor joining and maximum likelihood phylogenetic trees, respectively. Korean peninsula map was shown with grey guidelines of longitude and latitude. Black circles indicate sampling location of four Pseudostellaria samples (Kim et al. Citation2018, Citation2019; doi:10.1080/23802359.2019.1567279).
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780.
- Kim Y, Heo K-I, Lee S, Park J. 2018. Complete chloroplast genome sequence of the Pseudostellaria longipedicellata S. Lee, K. Heo & SC Kim (Caryophyllaceae). Mitochondrial DNA B. 3:1296–1297.
- Kim Y, Heo K-I, Lee S, Park J. 2019. Complete chloroplast genome sequence of the Pseudostellaria okamotoi Ohwi (Caryophyllaceae). Mitochondrial DNA B. 4:174–175. https://www.tandfonline.com/doi/full/10.1080/23802359.2019.1567279
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35:1547–1549.
- Kwiatkowska D. 1999. Formation of pseudowhorls in Peperomia verticillata (L.) A. Dietr. shoots exhibiting various phyllotactic patterns. Ann Bot. 83:675–685.
- Lee S, Heo K-I, Kim S-C. 2012. A new species of Pseudostellaria (Caryophyllaceae) from Korea. Novon. 22:25–31.
- Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv preprint arXiv:13033997.
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The sequence alignment/map format and SAMtools. Bioinformatics. 25:2078–2079.
- Ohwi J. 1937. The Revision of the genus Pseudostellaria. Jap J Bot. 9:95–105.
- Ōi J, Meyer FG, Walker EH. 1965. Flora of Japan.
- Park C. 2007. The genera of vascular plants of Korea. Seoul: Academic Publishing; p. 1482. ISBN. 745696222.
- Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829.
- Zhang SD, Jin JJ, Chen SY, Chase MW, Soltis DE, Li HT, Yang JB, Li DZ, Yi TS. 2017. Diversification of Rosaceae since the late cretaceous based on plastid phylogenomics. New Phytol. 214:1355–1367.
