Abstract
Thymallus brevirostris is a specific grayling species that naturally occurs in Mongolia and Russia in Asia. The complete mitochondrial genome of the Mongolian grayling T. brevirostris was determined (Accession number MH027384) with a total length of 16,653 nucleotides, of which 15,585 nucleotides are coding DNA and 1068 nucleotides are non-coding DNA. It has the common feature with those of other grayling with respect to genome structure and gene arrangement. It contained 13 protein-coding genes, 2 ribosomal RNA genes, 22 transfer tRNA genes, and 1 control region (D-loop region). The complete mitochondrial genome of Mongolian grayling T. brevirostris provides an important data set for further study on its classification.
As a kind of small economic fish, which belongs to Thymallus, Salmonidae, Salmoniformes, Protacanthopterygii, Euteleostei, Thymallus brevirostris is a specific grayling species that naturally occurs in the inland waters in northwestern Mongolia, Russia, and Asia). Due to the gradual exhaustion of resources, grayling fish has become one of the most popular fish. However, there is still no study on the complete mtDNA sequences for T. brevirostris that has been reported. So, it is important to obtain the whole mitogenome of T. brevirostris for further study. The specimen of T. brevirostris, named as Thybre-01 based on the phylogenetic analysis, was collected from Khoton Lake, Mongolia (48°65′N, 88°22′E) in 2016 and stored in College of Life Sciences, Linyi University, Linyi, China. Genomic DNA was extracted from Mongolian grayling muscle according to Liu et al. (Citation2012, Citation2014). The mitochondrial genome was amplified using 25 pars of primers, designed based on mitogenome sequence of the arctic grayling, T. arcticus arcticus (Accession number KJ866481) and the Xinjiang arctic grayling, T. arcticus grubei (Accession number LC168675). Protein-coding genes were analyzed by ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/ gorf.html) using the invertebrate mitochondrial code. The tRNA genes were identified by ARWEN (Laslett & Canback Citation2008) and tRNA-scan SE (Lowe & Eddy Citation1997)
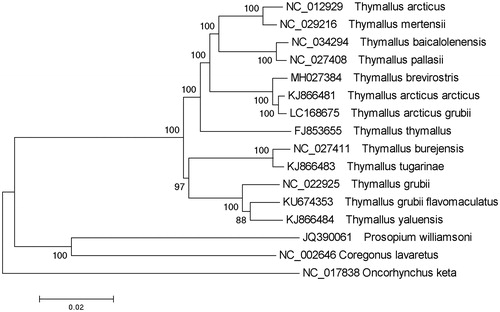
The complete T. brevirostris mitochondrial genome (Accession number MH027384) was 16,653 nucleotides long, of which 15,585 nucleotides are coding DNA, and 1068 nucleotides are non-coding DNA. The mitochondrial genome of T. brevirostris was found to contain 13 protein-coding genes, 2 ribosomal RNA genes, 22 transfer RNA genes, and 1 control region. Its arrangement and composition is also comparable to other vertebrates (Liang et al. Citation2012; Liu et al. Citation2013; Ma et al. Citation2015; Shan and Liu Citation2015; Xu et al. Citation2015; Balakirev et al. Citation2016, Citation2017). A phylogenetic tree was constructed based on the comparison of the complete mitochondrial genome sequences with other Thymallus species using the neighbour-joining method (). There were 10 overlapping regions (total of 65 bp) and 7 intergenic spacers (total 27 bp) among the genes. The overall base composition was 27.98% A, 27.11% T, 27.52% C, 17.39% G, with an AT content of 55.09%, which was similar with T. a. grubei (AT 55.18%) and T. thymallus (AT 55.15%). To investigate the nucleotide bias, skew for a given strand was calculated as (A–T)/(A + T) or (G–C)/(G + C) (Perna and Kocher Citation1995). The AT and GC skews for the T. brevirostris mitochondrial genome were 0.016 and -0.225, respectively; this finding indicated that the strand that encoded genes contained more A and C than T and G and this skew was evidence of codon usage bias. All 13 protein-coding genes identified in the T. brevirostris mitochondrial genome, started with the orthodox initiation codon ATG, except for COX1, which began with GTG. The ORFs of six protein-coding genes, ND1, COX1, ATP8, ATP6, NAD4L, and NAD5, ended with a TAA codon, and the remaining genes ended with incomplete stop codon T or AT. The length of 12S rRNA and 16S rRNA was 947 bp and 1678 bp, respectively. The intergenic region between 12S rRNA and 16S rRNA was found to be 72 bp and to contain only one tRNA (tRNA-val). The lengths of 22 tRNA coding genes range in the size from 67 to 75 nucleotides. The control region was located between tRNA-pro and tRNA-phe with a length of 1003 bp. The data would facilitate further investigations of phylogenetic relationships within Thymallus.
Figure 1. A phylogenetic tree constructed based on the comparison of complete mitochondrial genome sequences of the grayling, T. brevirostris and other 12 species of Thymallus genus. They are T. thymallus (European grayling), T. pallasii (East Siberian grayling), T. arcticus (Arctic grayling), T. tugarinae (Lower Amur grayling), T. burejensis (Bureya grayling), T. grubii (Amur grayling), T. arcticus arcticus (Arctic grayling), T. arcticus grubei (Xinjiang arctic grayling), T. yaluensis (Yalu grayling), T. mertensii (Kamchatka grayling), T. grubii flavomaculatus (Yellow-spotted grayling), and T. baicalolenensis (Baikal black grayling). Oncorhynchus keta, Coregonus lavaretus, and Prosopium williamsoni are used as an outgroup. Genbank accession numbers for all sequences are listed in the figure. The numbers at the nodes are bootstrap percent probability values based on 1000 replications.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Funding
References
- Balakirev ES, Romanov NS, Ayala FJ. 2016. Complete mitochondrial genome of the yellow-spotted grayling Thymallus flavomaculatus (Salmoniformes, Salmonidae). Mitochondr DNA B. 1:289–290.
- Balakirev ES, Romanov NS, Ayala FJ. 2017. Complete mitochondrial genome of the Kamchatka grayling Thymallus mertensii (Salmoniformes, Salmonidae). Mitochondr DNA A. 28:135–136.
- Laslett D, Canback B. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24:172–175.
- Liang HW, Hu GF, Li Z, Zou G W, Liu XL. 2012. Mitochondrial DNA sequence of yellow catfish (Pelteobagrus fulvidraco). Mitochondrial DNA. 23:170–172.
- Liu YG, Guo YG, Hao J, Liu LX. 2012. Genetic diversity of swimming crab (Portunus trituberculatus) populations from Shandong peninsula as assessed by microsatellite markers. Biochem Syst Ecol. 41:91–97.
- Liu YG, Kurokawa T, Sekino M, Tanabe T, Watanabe K. 2013. Complete mitochondrial DNA sequence of the ark shell Scapharca broughtonii: an ultra-large metazoan mitochondrial genome. Comp Biochem Physiol Part D. 8:72–81.
- Liu YG, Liu LX, Xing SC. 2014. Development and characterization of thirteen polymorphic microsatellite loci in the Cynoglossus semilaevis. Conserv Genet Resour. 6:683–684.
- Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25:955–964.
- Ma B, Jiang H, Sun P, Chen J. 2015. Complete mitochondrial genome of Thymallus grubii (Salmonidae: Thymallinae). Mitochondrial DNA. 26:815–816.
- Perna NT, Kocher TD. 1995. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J Mol Evol. 41:353–358.
- Shan WJ, Liu YG. 2015. The complete mitochondrial DNA sequence of the cape hare Lepus capensis pamirensis. Mitochondr DNA.
- Xu D, He CQ, Li QH, He J, Ma HM. 2015. The complete mitochondrial genome of the Daweizi pig. Mitochondr DNA. 26:640–641.
- Yasuike M, Jantzen S, Cooper G, Leder E, Davidson W, Koop B. 2010. Grayling (Thymallinae) phylogeny within salmonids: complete mitochondrial DNA sequences of Thymallus arcticus and Thymallus thymallus. J Fish Biol. 76:395–400.
