Abstract
The complete chloroplast genome sequence of Chelidonium majus L. has been presented in this article. The complete chloroplast genome is 159,741 bp in length with 38.7% of GC content, containing a large single copy region (LSC) of 87,698 bp, and a small single copy region (SSC) of 18,573 bp, which were separated by a pair of 26,735 bp inverted repeat regions (IRs). The sequence contained 132 unique genes, including 37 tRNA, 8 rRNA, and 87 protein-coding genes. This plastid genome is the first report for the genus Chelidonium and will be useful data for developing markers for further studies on resolving the relationship within the Papaveraceae.
Chelidonium majus L., an herbaceous perennial plant which is widely distributed in Europe and Asia, commonly known as greater celandine, is the only species of the genus Chelidonium in the Papaveraceae family. Chelidonium majus has attracted research attention not only for its medicinal value but also for its well-known ant-dispersal habitat. Chelidonium majus is a typical myrmecochorus plant that has been investigated in several studies on ant-seed interactions (Gorb and Gorb Citation2000; Fischer et al. Citation2008; Servigne and Detrain Citation2008; Reifenrath et al. Citation2012; Deljanin et al. Citation2016). This study is the first report for the genus Chelidonium in Papaveraceae with complete chloroplast genome of C. majus.
Fresh young leaves of Chelidonium majus were collected in Beijing Badaling National Forest Park, China (N 40°20′46.01″, E 116°0′52.20″), under the natural conditions, and its voucher specimen (with collection numbers of J. He PH20170823) has been deposited in the Herbarium of Beijing Forestry University (BJFC). Total genome DNA was extracted using a modified protocol (Chen et al. Citation2014) and sent to Majorbio Company for next-generation sequencing using Illumina Hiseq Xten (http://www.majorbio.com). About 2 Gb high quality, 2 × 150 base pairs PE reads were obtained from high-throughput sequencing. We used map to reference function in Geneious 11.1.5 (Kearse et al. Citation2012) to exclude nuclear and mitochondrial reads using published plastid genome of Coreanomecon hylomeconoides (GenBank accession no. NC 031446) chloroplast genomes as the reference and used these filtered chloroplast reads for de novo assembly with Geneious R11 (Kearse et al. Citation2012). The assembled chloroplast sequences were then annotated using Plann (Huang and Cronk Citation2015) by referring to the relative group and we perfected the annotation by Geneious.
The chloroplast genome has a total length of 159,741 bp, including a large single copy region (LSC) of 87,698 bp, a small single copy region (SSC) of 18,573 bp, and a pair of 26,735 bp inverted repeat regions (IRs). The overall GC-content of the whole plastome genome is 38.7%. The sequence contains 132 unique genes, including 37 tRNA, 8 rRNA, and 87 protein-coding genes.
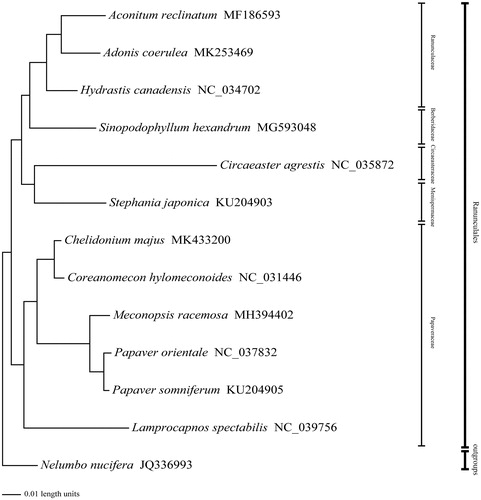
To clarify its phylogenetic position in the Papaveraceae, a Bayesian inference (BI) tree based on complete chloroplast genome sequences of 5 Papaveraceae species, 6 Ranunculales and Nelumbo nucifera as the outgroup from NCBI was reconstructed in MrBayes 3.2.3 (Ronquist and Huelsenbeck Citation2003). All the sequences were aligned by MAFFT v6.833 (Katoh et al. Citation2005), and the appropriate nucleotide substitution model for each sequence was according to the Akaike information criterion (AIC; Posada and Buckley Citation2004) by using jModeltest (Posada Citation2008). In the phylogenetic tree in , the posterior probability (PP) of all branch nodes is 1.00. As shown in , our research supports that Chelidonium majus and Coreanomecon hylomeconoides are clustered into one clade, which is sister to the clade of the genera Papaver (Zhou et al. Citation2018), Meconopsis, and Lamprocapnos. Meanwhile, the phylogenetic relationship in Papaveraceae was consistent with previous studies and this will be useful data for developing markers for further studies.
Disclosure statement
The authors declare there are no conflicts of interest and are responsible for the content.
Additional information
Funding
References
- Chen LY, Song MS, Zha HG, Li ZM. 2014. A modified protocol for plant genome DNA extraction. Plant Diversity Resour. 36:375–380.
- Deljanin M, Nikolic M, Baskic D, Todorovic D, Djurdjevic P, Zaric M, Stankovic M, Todorovic M, Avramovic D, Popovic S. 2016. Chelidonium majus crude extract inhibits migration and induces cell cycle arrest and apoptosis in tumor cell lines. J Ethnopharmacol. 190:362–371.
- Fischer RC, Richter A, Hadacek F, Mayer V. 2008. Chemical differences between seeds and elaiosomes indicate an adaptation to nutritional needs of ants. Oecologia. 155:539–547.
- Gorb E, Gorb S. 2000. Effects of seed aggregation on the removal rates of elaiosome-bearing Chelidonium majus and Viola odourata seeds carried by Formica polyctena ants. Ecological Research. 15:187–192.
- Huang DI, Cronk QC. 2015. Plann: a command-line application for annotating plastome sequences. Appl Pl Sci. 3:1500026.
- Katoh K, Kuma K, Toh H, Miyata T. 2005. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 33:511–518.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28:1647–1649.
- Posada D, Buckley TR. 2004. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol. 53:793–808.
- Posada D. 2008. jModelTest: phylogenetic model averaging. Mol Biol Evol. 25:1253–1256.
- Reifenrath K, Becker C, Poethke HJ. 2012. Diaspore trait preferences of dispersing ants. J Chem Ecol. 38:1093–1104.
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics. 19:1572–1574.
- Servigne P, Detrain C. 2008. Ant-seed interactions: combined effects of ant and plant species on seed removal patterns. Insectes Sociaux. 55:220–230.
- Zhou JG, Cui YX, Chen XL, Li Y, Xu ZC, Duan BZ, Li YH, Song JY, Yao H. 2018. Complete chloroplast genomes of Papaver rhoeas and Papaver orientale: molecular structures, comparative analysis, and phylogenetic analysis. Molecules. 23:437.